Recent advances in quantum biology have sparked a paradigm shift in our understanding of Alzheimer’s disease, particularly concerning the role of amyloid fibrils—protein structures typically seen as central players in neurodegenerative diseases. Conventional approaches to tackling Alzheimer’s have focused primarily on the amyloid hypothesis, which posits that the presence of these fibrils is a fundamental cause of the disease. This has led to a significant investment in developing treatments aimed at reducing amyloid levels in affected individuals. However, emerging research presents a contrasting view that questions this longstanding assumption.
While amyloid fibrils are indeed associated with Alzheimer’s and other forms of dementia, studies have revealed that numerous individuals with high levels of amyloids do not exhibit any symptoms of cognitive decline. This inconsistency raises critical questions about the historical narrative surrounding the disease and its underlying biological processes. Current treatment regimens targeting amyloid reduction have also fallen short of delivering the expected benefits, signaling an urgent need for fresher perspectives and approaches to understanding Alzheimer’s.
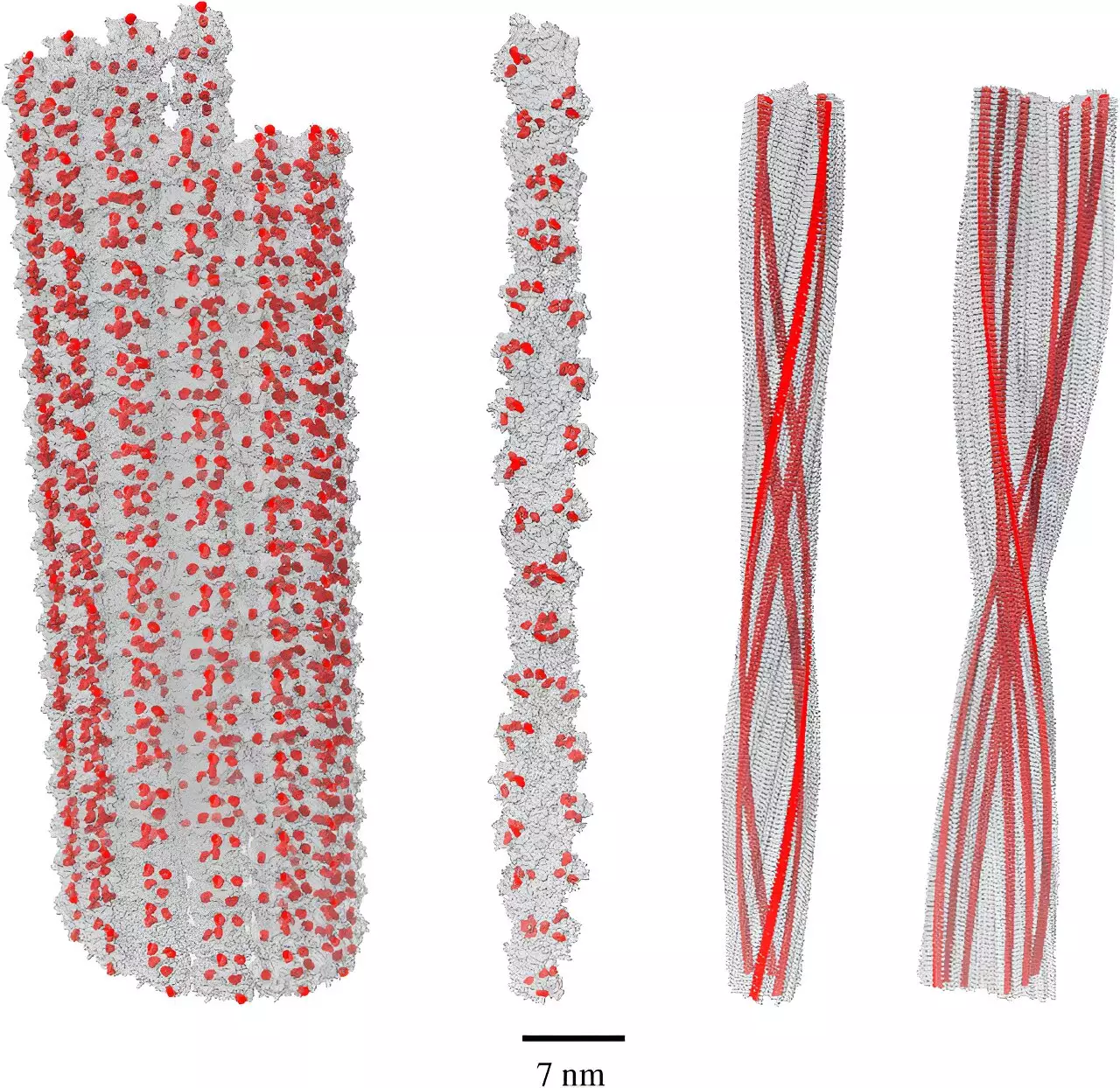
A pivotal study conducted by Dr. Philip Kurian and his team at the Quantum Biology Laboratory at Howard University has uncovered a unique quantum effect that might redefine our understanding of amyloid fibrils and their interaction with oxidative stress in the human brain. The research revealed that a phenomenon known as single-photon superradiance could occur in networks formed by the amino acid tryptophan. This effect allows a cooperative network of molecules to efficiently absorb harmful high-energy UV photons and re-emit them as less harmful energy forms.
The implications of these findings are profound. Previously thought of solely in a detrimental light, amyloid fibrils may play a protective role by helping to mitigate oxidative stress, a known contributing factor to Alzheimer’s pathology. The recent work indicates that the potential for superradiant effects is significantly enhanced in amyloid fibrils—up to five times higher than that observed in individual tryptophan molecules. This revelation shifts the narrative surrounding amyloid aggregates from being mere agents of neurodegeneration to potentially protective structures in response to environmental stressors within the brain.
This emerging perspective positions amyloid fibrils as a possible adaptive reaction to the toxic environment created by oxidative stress rather than a causative factor in Alzheimer’s development. High levels of oxidative stress, marked by the accumulation of free radicals, can have deleterious effects on cellular structures and processes, leading to a cycle of damage and dysfunction. The ability of amyloid fibrils to absorb and downconvert high-energy photons provides a mechanism through which cells may protect themselves, ultimately reshaping our understanding of their role in neurodegenerative diseases.
Professor Lon Schneider, a respected authority in Alzheimer’s research, has acknowledged the significance of Kurian’s findings, emphasizing their transformative potential in unraveling the pathophysiology of Alzheimer’s. The mounting evidence challenges the conventional notion that treatment strategies should primarily target amyloid levels within the brain. Instead, this research advocates for a broader inquiry into how amyloid aggregation might represent an evolved response to combat oxidative stress rather than its primary cause.
Moving forward, Dr. Kurian aims to validate these groundbreaking findings through experimental work, emphasizing the need for collaborative efforts between quantum physics and biology. He encourages fellow researchers to embrace a multifaceted view of life sciences, integrating perspectives from various disciplines such as computational biology, photophysics, and quantum systems.
The revelation that interactions between light and biological systems hold significant implications offers a compelling avenue for further exploration. As we deepen our understanding of how quantum effects may contribute to biological processes, there could be transformative potentials for designing innovative therapeutic strategies for Alzheimer’s and other neurodegenerative disorders.
Mr. Hamza Patwa, a senior undergraduate intern who contributed to the research, encapsulated the essence of this interdisciplinary approach. He argued that true scientific inquiry thrives on crossing disciplinary boundaries and championing collaboration to unravel the complex nature of life and disease.
As we stand on the precipice of a new era in Alzheimer’s research, the integration of quantum biology into the conversation may illuminate pathways toward more effective treatments. By revising our perceptions of amyloid fibrils and their function in the human brain, we open the door to novel therapeutic possibilities. The insights gleaned from studies like Kurian’s not only enrich our scientific understanding but also inspire hope for improved strategies and outcomes in the ongoing battle against Alzheimer’s disease.
In embracing the intricate dance of light and matter within biological systems, researchers may soon uncover the keys to deciphering one of the greatest health challenges of our time.

Leave a Reply