In the ever-evolving landscape of material science, high entropy oxides (HEOs) have emerged as a prominent focus due to their remarkable electrochemical properties and vast potential for applications in technology. The recent study published in the Journal of the American Chemical Society sheds light on how various synthesis techniques can significantly influence both the structural and functional characteristics of these intricate materials. This groundbreaking research not only emphasizes the importance of synthesis methods in determining material properties but also opens new avenues for optimizing HEOs for use in electronic devices and energy systems.
At the heart of the study is a specific high entropy oxide characterized by a spinel crystal structure. This unique material comprises a blend of five distinct transition metal oxides, showcasing the rich compositional variety inherent to HEOs. According to Alannah Hallas, a leading materials scientist involved in the research, the versatility amalgamated in high entropy systems grants researchers a plethora of options when it comes to synthesizing and tailoring these materials. This flexibility not only promises enhanced function but also invites creativity in structuring the oxides to meet specific technological needs.
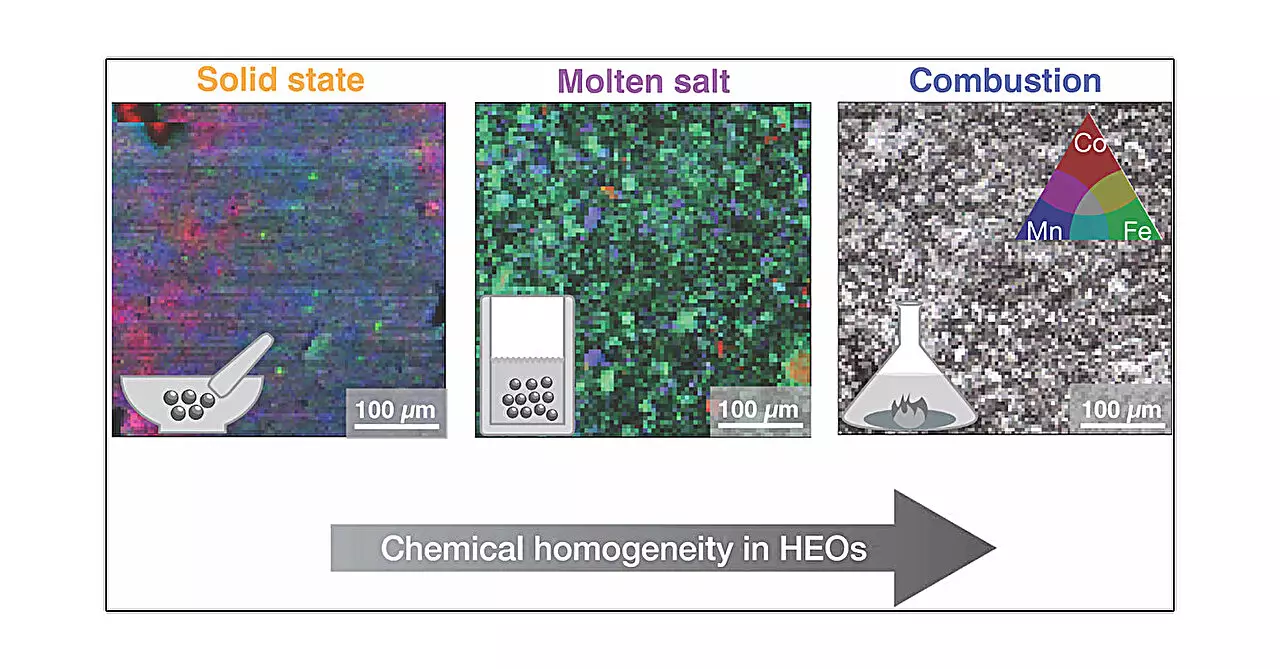
The investigation painstakingly employed five different synthesis methods, namely solid-state, high-pressure, hydrothermal, molten salt, and combustion syntheses. Each of these techniques follows a unique protocol regarding heating and cooling, introducing varied chemical environments and operational conditions. For instance, in the solid-state synthesis, metal oxides are blended and heated, similar to culinary baking. In contrast, the high-pressure method introduces additional pressure during the heating stages, which is known to dramatically alter material formation dynamics.
Furthermore, the hydrothermal approach emulates natural processes by using pressurized water to facilitate the growth of metal salt crystals, embodying a process comparable to mineral formation at great depths within the Earth. The molten salt method presents its own unique dynamics, where melted salts create a viscous medium that crystalizes upon cooling, while the combustion synthesis technique utilizes a rapid exothermic reaction to achieve the desired materials quickly. This varied toolkit of synthesis routes underscores the intricate complexity of creating HEOs and highlights how slight variations in methods can lead to radically different outcomes.
One of the central findings of this study is the pronounced impact of synthesis technique on the local structural and microstructural properties of HEO samples. While the overall average structure remained consistent irrespective of the synthesis method, the microstructural variations unveiled significant differences. The combustion synthesis method was found to yield the most homogeneous samples, marking a critical result regarding how specific synthesis choices can dictate the performance characteristics of high entropy oxides.
Lead author Mario Ulises González-Rivas articulated this notion effectively, emphasizing that the varying mechanisms driving material synthesis were crucial. This insight not only affects fundamental understanding but also lays a foundation for enhancing the performance of HEOs in applied contexts, particularly in addressing pressing energy challenges that the world faces today.
The implications of this study echo deeply through the field of materials science, suggesting a new optimization axis for implementing high entropy oxides in relevant technologies. The exploration into synthesis methods may very well appoint researchers with the refined understanding needed to fine-tune these materials for precise applications, thereby enhancing their integration into everyday electronic devices and energy systems.
The collaborative efforts of the research team, comprising experts from the University of British Columbia and the Max Planck Institute, have provided invaluable insights into the structural intricacies of high entropy oxides. By carefully analyzing the synthesis methods and their repercussions on the resultant materials, they have not only advanced the scientific discourse on HEOs but also set the stage for future innovations that could propel technology towards more efficient, sustainable solutions. The journey of uncovering the yet uncharted territories of high entropy oxides continues, promising a future where these materials could play a pivotal role in meeting global energy demands.

Leave a Reply