The immunoproteasome is a specialized form of the proteasome, a complex responsible for degrading unneeded or damaged proteins within cells. This enzymatic powerhouse plays a pivotal role in the immune response, as it helps in processing and presenting antigens from pathogens like bacteria and viruses to immune cells, effectively training the immune system to recognize threats. However, when the immunoproteasome becomes hyperactive, it can mistakenly target and attack the body’s own cells, contributing to autoimmune diseases and various inflammatory conditions. The challenge for researchers has long been to find a way to selectively inhibit this specific proteasome variant to manage such diseases without hampering other essential cellular processes.
Challenges in Selective Inhibition
The quest for selective inhibitors of the immunoproteasome has proven elusive. Traditional proteasome inhibitors often affect all proteasome types within cells, leading to undesirable side effects that can complicate treatment outcomes. Given the crucial functions of other proteasome forms in maintaining cellular homeostasis—such as protein recycling and waste management—it’s imperative that any therapeutic approach designed to inhibit the immunoproteasome does so without adversely affecting these other variants. This delicate balance highlights the need for a new class of drugs that can specifically target the immunoproteasome, reducing the risk of off-target effects that may harm normal cellular functions.
Recent breakthroughs in the lab of Helge Bode at the Max Planck Institute for Terrestrial Microbiology are opening up exciting avenues for the development of more precise therapeutics. These advancements involve manipulating naturally occurring bacterial substances to generate a new class of drugs that target the immunoproteasome selectively. This novel approach draws upon synthetic biology techniques to design and synthesize hybrid compounds that combine elements from different classes of natural products, merging peptides and polyketides that hold promise for immune modulation.
Bode’s research team, collaborating with experts from Technical University Munich and the University of Duisburg-Essen, has developed what they refer to as “XUT technology.” This innovative methodology leverages specific docking sites in thiolation domains present in both non-ribosomal peptide synthetases (responsible for peptide synthesis) and polyketide synthases (responsible for polyketide formation). The ability to fuse these two types of enzymes allows for the rapid assembly of peptide-polyketide hybrids, offering a versatile platform for drug development.
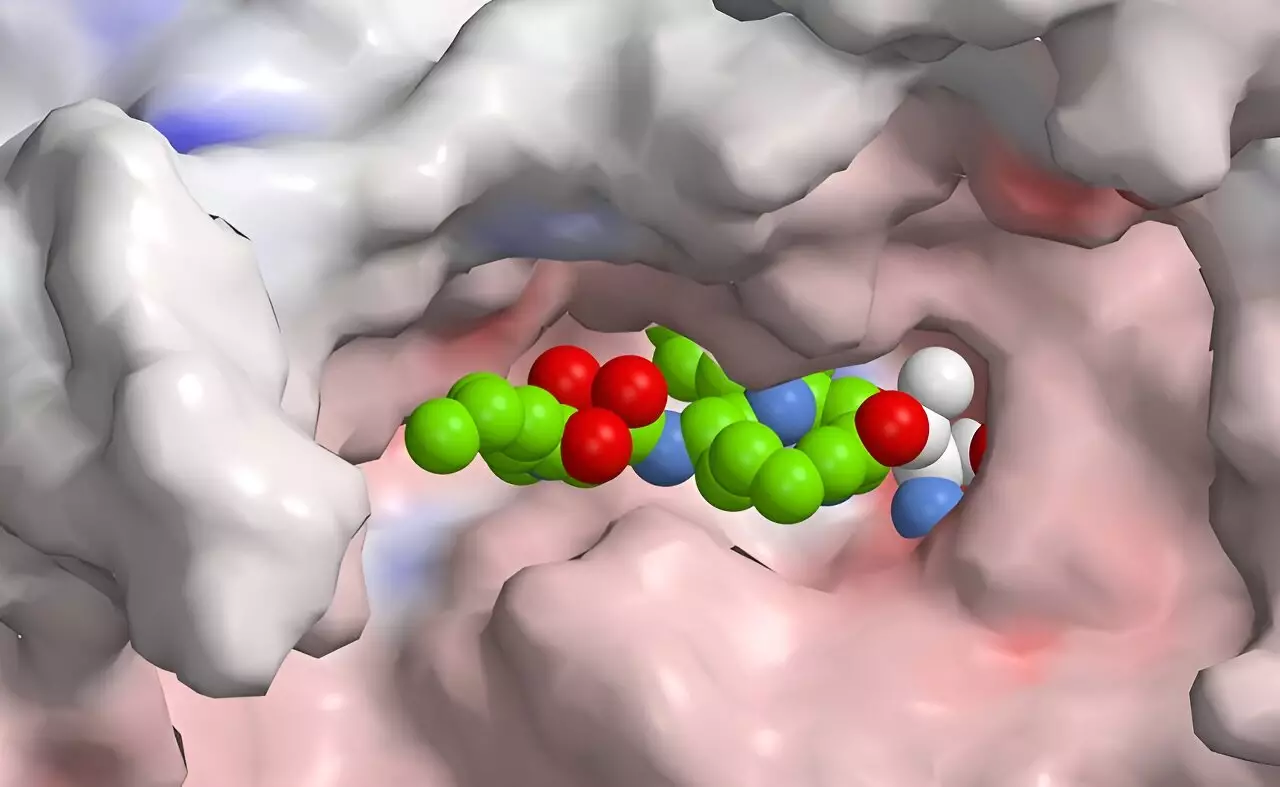
Syrbactins, a class of natural products discovered in bacteria that target plant and insect cells, provide further inspiration for this research direction. These compounds inhibit proteasome function, leading to cell death by effectively overwhelming cellular waste disposal systems. Their potential as anticancer agents arises from their ability to induce apoptosis selectively in tumor cells. The integration of knowledge from natural product chemistry and synthetic pathways offers a pathway to modifying syrbactins and generating more selective inhibitors specific to the human immunoproteasome.
The recent study demonstrates that while the initial results do not yet yield a drug with the requisite selectivity, they underscore the potential for rational drug design. By employing computational modeling and high-throughput screening methods, researchers aim to iteratively refine these compounds, enhancing their selectivity while minimizing side effects.
The development of selective immunoproteasome inhibitors represents a significant advancement in the therapeutic landscape for autoimmune diseases and inflammation. As researchers like Helge Bode and his team continue to innovate using synthetic biology and natural compounds, the potential to create drugs that target specific cellular pathways without affecting overall cellular function grows ever more tangible. This targeted approach promises a future where the precision of medical interventions aligns more closely with the complexities of human health, paving the way for safer and more effective treatments in biomedicine. The evolution of drug design reflects a broader understanding of both the molecular mechanisms at play and the importance of specificity within the immune response, signaling a promising horizon for both researchers and patients alike.

Leave a Reply