In the intricate web of biological processes, proteins play an indispensable role, acting as orchestrators of growth, metabolism, and various cellular functions. One such protein, myo-inositol-1-phosphate synthase (MIPS), has recently captured the attention of researchers due to its dynamic nature. Traditionally viewed as static entities, proteins can exhibit significant structural changes upon activation, a phenomenon that is crucial to their functionality. MIPS undergoes a transformation from a disordered structure into a more defined, functional form, marking its pivotal role in the synthesis of inositol, often referred to as vitamin B8, despite the body’s ability to produce it.
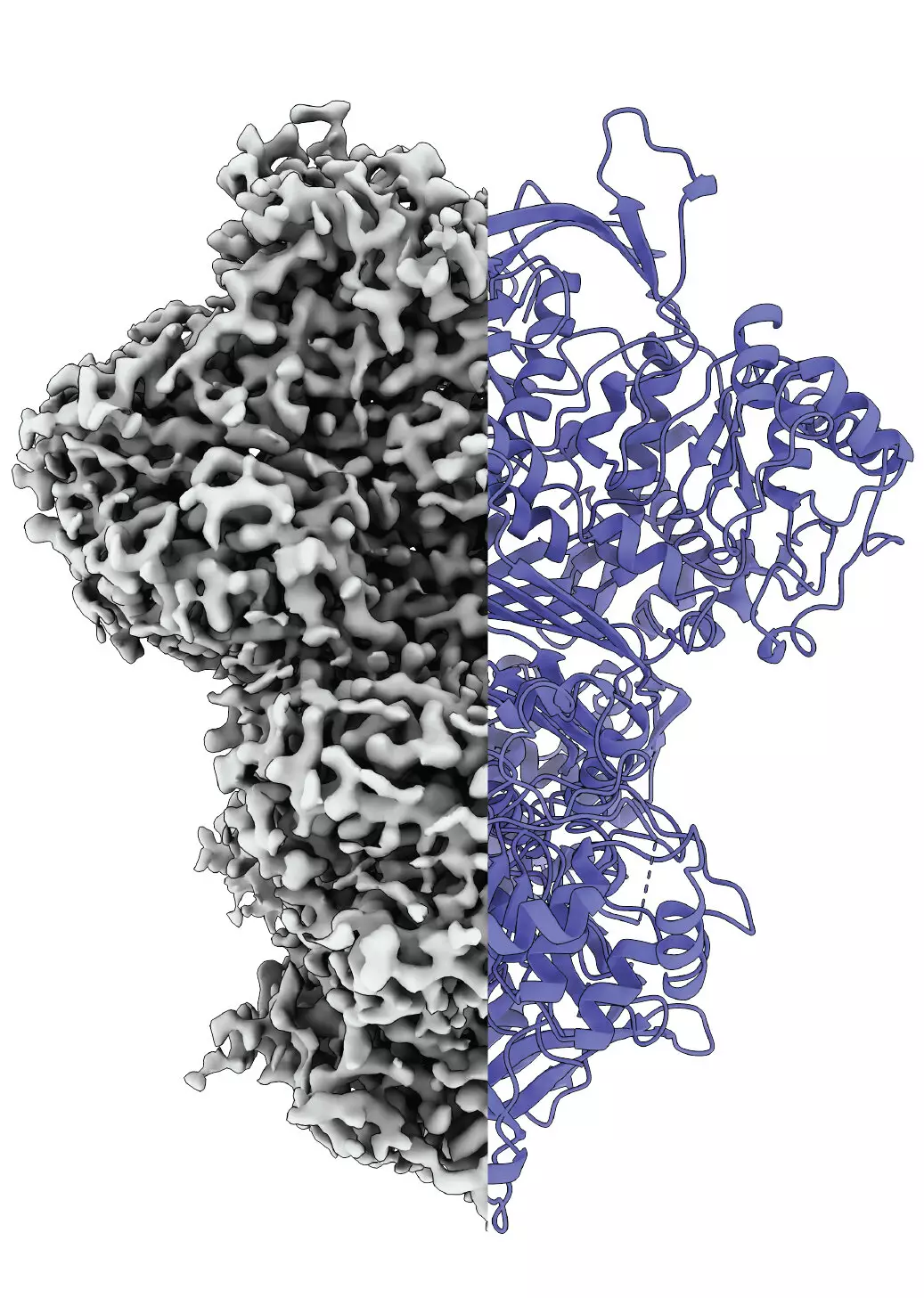
Researchers from Martin Luther University Halle-Wittenberg (MLU) and the National Hellenic Research Center in Greece have made a groundbreaking stride in understanding MIPS by observing its structural transitions in real time. This achievement is significant because the examination of proteins typically requires isolating them from their natural context, hindering the ability to study their behavior dynamically. Professor Panagiotis Kastritis and his team have developed innovative methods to analyze proteins under near-native conditions, allowing the disordered and ordered states of MIPS to be visualized during its functional cycle. This approach enables a more realistic understanding of protein behavior, shedding light on how MIPS contributes to inositol production.
MIPS is not merely another protein; it is part of an intricate metabolic pathway that contributes to inositol synthesis. This compound is essential for a variety of biological functions and is involved in signaling pathways, making it critical for overall health. Despite not being classified as a true vitamin since the body can synthesize it, inositol’s myriad roles necessitate an efficient and responsive synthesis process—something that MIPS is integral to. Understanding MIPS’s activation and structural adaptations can provide invaluable insights into nutritional biochemistry and metabolic disorders, where inositol’s availability may impact health.
Employing advanced cryo-electron microscopy, the research team observed that MIPS exists in three distinct states: an unordered state, an ordered state, and a less-defined intermediary state. The discovery of this intermediary state is particularly intriguing. While the precise role of this state remains elusive, it may play a significant function, potentially aiding in water absorption that could facilitate subsequent biochemical reactions. Kastritis’s team acknowledges the complexity of these interactions and sees this finding as a catalyst for further exploration into the activation mechanisms that drive such structural changes.
While the focus has been on MIPS, the implications of this research are extensive. The team’s analysis extended to over 340 isomerases, a broader class of proteins to which MIPS belongs, revealing behavior that aligns with the dynamics observed in MIPS. This finding suggests that the phenomena of ordered and disordered states may be a common thread among similar proteins, opening doors to comprehensive studies on protein functionality. Understanding these dynamics has substantial implications, particularly in the realm of therapeutic development—better knowledge of metabolic pathways and protein actions could pave the way for innovative treatments.
Kastritis concludes that this work constitutes a vital first step toward unraveling the complexities of protein structures in metabolic processes. As the team delves deeper into the realms of protein dynamics, the prospects for unlocking new therapeutic strategies to combat metabolic disorders appear increasingly promising. The continuing exploration of proteins like MIPS, with their multifaceted roles in metabolism and beyond, continues to shape the field of biochemistry and potentially revolutionize our understanding of health and disease management. The quest for knowledge around such proteins is not just scientific inquiry—it could transform the way we approach therapeutic interventions in the future.

Leave a Reply