Ammonia is a pivotal compound in various industries, notably in agriculture as a key ingredient in fertilizers, as well as in the synthesis of numerous chemical products. With a staggering global market that evaluates its production at approximately 175 million metric tons valued at $67 billion, the importance of ammonia in the world cannot be overstated. Its role extends beyond traditional uses, gradually becoming a significant player in the burgeoning hydrogen economy due to its capabilities as a high-energy-density carrier. However, conventional ammonia production approaches, particularly the Harber-Bosch process, present serious environmental concerns. This well-established method is not only energy-intensive but also a significant contributor to CO2 emissions, which must be addressed to ensure sustainability in industrial practices.
In light of these ecological challenges, innovative research conducted by a team led by Hao Li from Tohoku University has unearthed a promising alternative pathway for ammonia synthesis—electrochemical conversion of nitrates. This groundbreaking technique, discussed in the journal Advanced Science, represents a paradigm shift from the nitrogen reduction reaction (NRR), which infamously struggles with the stability of the N=N triple bond in molecular nitrogen (N2). Conversely, the focus on nitrate reduction (NO3RR) presents a more feasible and environmentally friendly approach to producing ammonia. Nitrates are not only easier to handle due to their lower dissociation energy but also address a significant environmental issue: their accumulation in aquatic systems, which can lead to detrimental ecological impacts.
Catalyst Innovations and Enhanced Yields
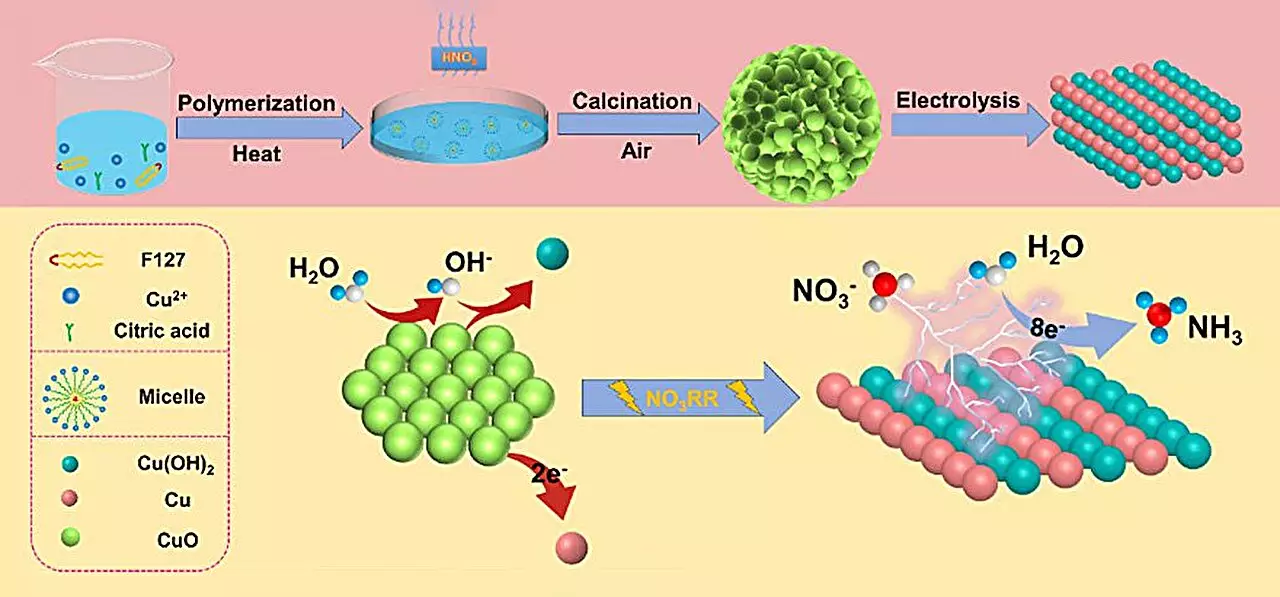
At the heart of this research is the creation of a novel copper (II) oxide (CuO) catalyst, meticulously designed with oxygen-rich vacancies and small particle stacking. The results are impressive, showcasing an ammonia yield of 15.53 mg h-1 mgcat-1 and achieving a remarkable Faraday efficiency of 90.69% when tested in a neutral electrolyte at a voltage of -0.80 V. The catalyst’s enhanced performance is attributed to unique structural and phase changes during the reduction process. Insights from Qiuling Jiang, a co-author and Ph.D. student involved in the research, highlight that the transformation from CuO to a mixed Cu/Cu(OH)2 structure during the reaction is pivotal for achieving heightened catalyst performance. This occurrence not only amplifies the number of active sites available for catalysis but also facilitates improved electron transfer at the electrode surface.
The utilization of density functional theory (DFT) has provided additional clarity regarding the catalytic mechanisms at play. These advanced calculations demonstrated that the formation of Cu(OH)2 significantly lowers the energy barrier associated with nitrate adsorption, rendering the overall process energetically favorable. Moreover, the Cu(OH)2 phase acts as a regulatory element, effectively dissuading the competing hydrogen evolution reaction (HER) while enhancing electron interactions on the Cu (111) crystal surfaces. This comprehensive approach allows for a better understanding of how adjustments in reaction conditions can optimize catalyst performance, ultimately steering the production of ammonia toward more efficient avenues.
Future Directions in Sustainable Ammonia Production
As the research progresses, the team is dedicated to delving deeper into the phase transition dynamics of the catalysts during the ammonia reduction process. The goal is to refine these innovative catalysts further, augmenting their stability, activity, and selectivity. Each enhancement brings the industrial sector one step closer to realizing sustainable methods for ammonia production, essential for reducing the environmental footprint associated with traditional practices. The implications of this research extend beyond just ammonia synthesis; they paint a hopeful picture for the larger energy landscape, potentially paving the way for the integration of cleaner technologies in the fight against climate change.
The shift toward electrochemical nitrate reduction presents a promising future for ammonia production. By leveraging advanced catalysts and understanding underlying chemical mechanisms, researchers like Li and his team are not only contributing to industrial efficiency but also fostering environmental stewardship—an imperative for future generations. This paradigm shift in ammonia synthesis could indeed signify a remarkable step forward in achieving a sustainable and eco-friendly industrial landscape.

Leave a Reply