Recent advancements in the field of electrocatalysis could pave the way for more efficient and sustainable energy solutions. A novel study highlights the development of an erbium (Er)-doped cobalt oxide (Co3O4) electrocatalyst, aiming to improve the oxygen evolution reaction (OER) in acidic environments. Conducted by researchers led by Tianyi Wang from Tohoku University’s Advanced Institute for Materials Research, this innovative catalyst presents a compelling alternative to traditional costly noble metal-based catalysts, showcasing both enhanced performance and stability as reported in the prestigious journal ACS Catalysis.
The Challenge of Oxygen Evolution Reactions
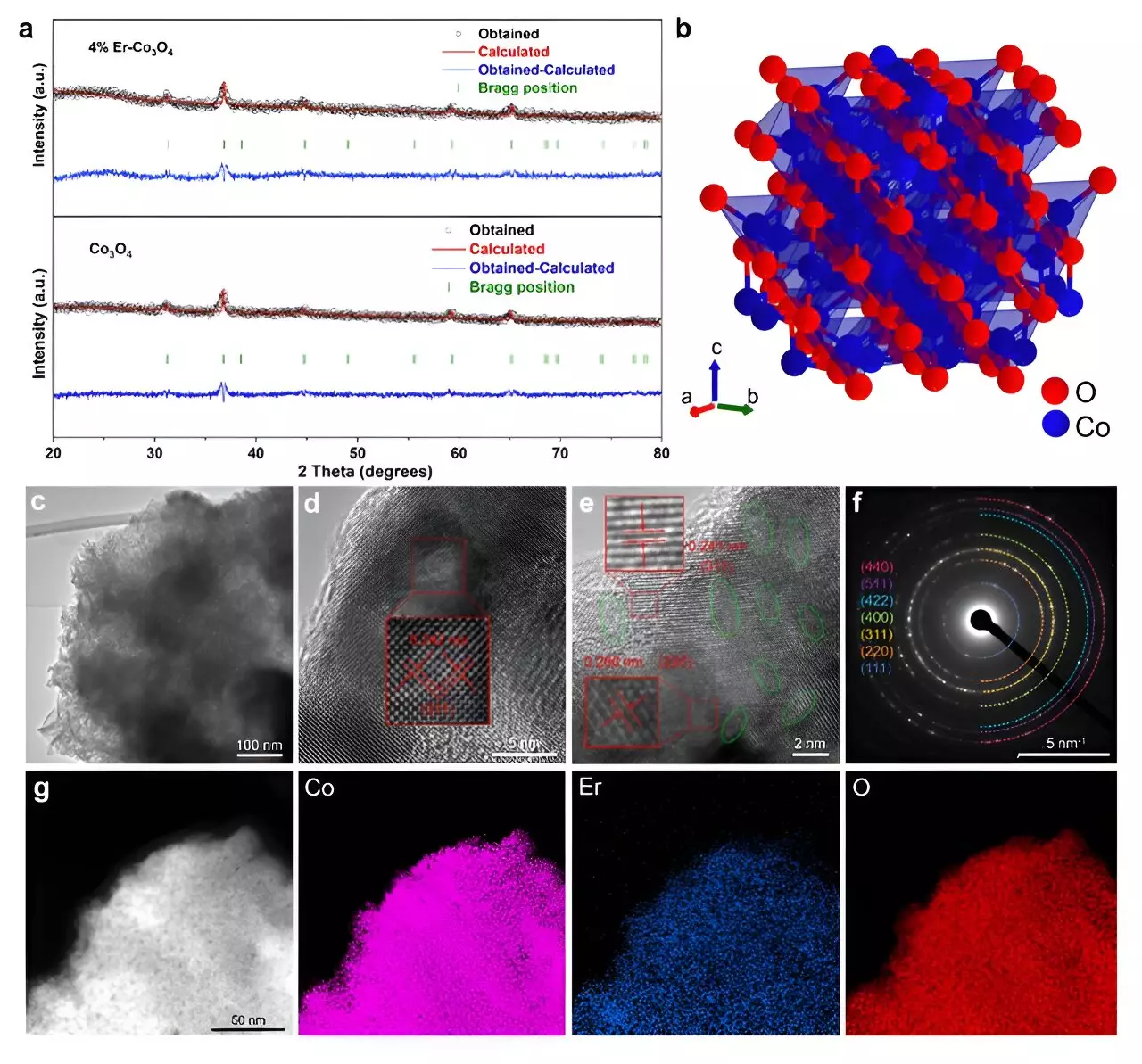
Oxygen evolution is a pivotal reaction in various energy conversion processes, including water splitting, which forms the foundation for hydrogen production as a clean energy source. In acidic environments, reliable catalysts are essential to facilitate these reactions at an acceptable rate. Historically, cobalt-based catalysts have been considered effective; however, their performance has often been hampered by limitations associated with their spinel crystal structure. In light of these challenges, the introduction of Er represents a transformative step in catalysis.
Mechanisms of Enhanced Performance
The essence of the research lies in the doping of cobalt oxide with a modest amount of erbium. Doping, a common technique in material science, can significantly alter the properties of a catalyst. The team discovered that the 4% Er-doped Co3O4 catalyst attained a remarkable overpotential of just 321 mV at a current density of 10 mA cm-2, showcasing stability that exceeded 250 hours. Such findings put it on par with or better than more expensive alternatives like iridium-based catalysts.
Advanced analytical techniques, such as microkinetic modeling and density functional theory (DFT) calculations, were employed to investigate the catalyst’s unique behavior. It was identified that the incorporation of Er helped to create more active sites within the catalyst’s crystalline framework, thereby enhancing the ratio of Co3+ to Co2+ ions. This modification encourages the formation of oxygen vacancies, which are essential for facilitating the OER process.
One of the remarkable aspects of this study is how the researchers have effectively illustrated the benefits of erbium doping through an analogy. As described by Hao Li, an associate professor at WPI-AIMR, envisioning the catalyst as a road system serves as a powerful metaphor. By adding lanes—represented by the oxygen vacancies—the flow of oxygen intermediates becomes smoother, thus accelerating the overall reaction process. This vivid analogy allows for a clearer understanding of how structural enhancements can lead to superior catalytic efficiency.
Structural Insights Through Spectroscopy
Employing in situ Raman spectroscopy revealed that the augmented oxygen vacancies from Er incorporation predominantly appeared in the octahedral sites of the Co3O4 structure. Such vacancies are critical, as they facilitate the development of essential intermediate species that are imperative for the OER. Additionally, an enhanced ratio of Co3+/Co2+ ions boosts the availability of active sites required to anchor these intermediates, which ultimately leads to improved catalytic activity.
The implications of this study extend well beyond the immediate successes of the erbium-doped Co3O4. By demonstrating the substantial potential of non-precious metal doping, the researchers open avenues for the future design of high-performance, cost-effective catalysts. Such innovations could lead to further advancements in sustainable energy technologies and reduce reliance on high-cost precious metals, which are often limited in availability.
The work of Wang and his colleagues distinguishes itself as a pivotal step in the evolution of electrocatalysts. This research promises to contribute significantly to the ongoing effort to transition to more sustainable energy systems, embodying not just a technical victory, but a progressive outlook toward future energy solutions.

Leave a Reply