In the quest for sustainable industrial practices, the recent advances in electrochemical methods signal a pivotal shift towards more environmentally friendly production processes. A groundbreaking study spearheaded by scientists at Lawrence Livermore National Laboratory (LLNL) has unveiled an innovative approach to chemical manufacturing, utilizing thin film nickel anodes that enhance both the cleanliness and energy efficiency of chemical reactions. This new method promises to reshape traditional production paradigms by reducing environmental impact while improving overall efficacy.
At the heart of this new approach is the utilization of thin film technology, which significantly simplifies the complexity often associated with heterogeneous catalyst reactions. Traditional catalysts suffer from inconsistencies due to variations in surface texture and thickness, complicating mechanisms of reaction. By employing thin films, as explained by Aditya Prajapati, a postdoctoral researcher involved in this study, scientists can achieve a uniform catalytic surface, which leads to clearer insights into the behavior and effectiveness of catalysts. This precision allows for more robust investigations into catalytic processes, fostering significant advancements in chemical production techniques.
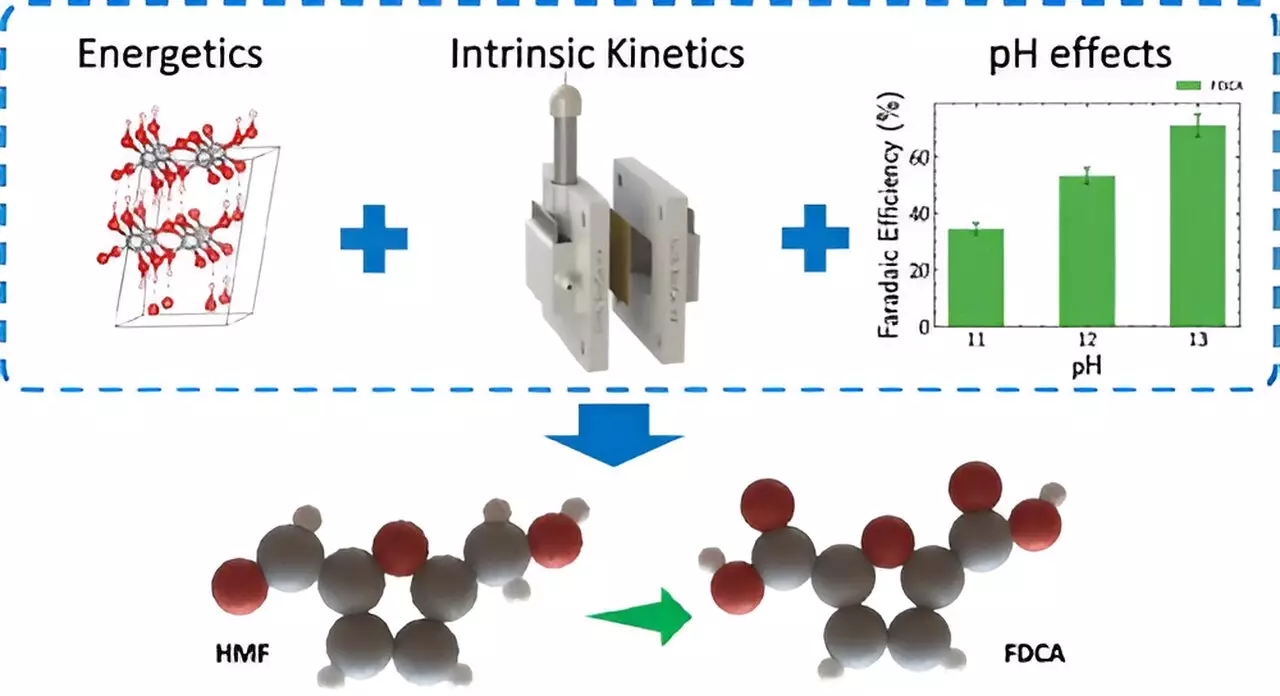
A critical innovation in this work is the shift from the conventional oxygen evolution reaction at the anode towards oxidation reactions of biomass. This change not only eliminates the inefficiencies that plague current electrolyzers but can also result in energy savings of over 50%. The researchers have specifically targeted the conversion of 5-Hydroxymethylfurfural (HMF) from biomass sources into 2,5-Furandicarboxylic acid (FDCA). FDCA is a promising intermediate for creating sustainable plastics, thus aligning chemical production with a broader sustainability agenda. Not only does this process reduce the reliance on fossil fuels, but it also mitigates harmful carbon emissions, positioning it as a cleaner alternative to traditional methods.
The implications of this electrochemical method extend beyond mere efficiency. By using renewable biomass as a feedstock, the process enhances the feasibility of a circular economy, where resources are reused and waste is minimized. As emphasized by corresponding author Nitish Govindarajan, this innovative technique avoids the high temperatures and toxic waste associated with traditional methods. Additionally, when powered by renewable energy sources, there’s a potential to achieve a zero-carbon footprint in chemical manufacturing, presenting an exciting avenue towards broader climate objectives.
The LLNL research team, which includes notable contributions from Wenyu Sun, Jeremy Feaster, and Sneha Akhade, collaborated with institutions such as Université de Montréal and the University of Bonn, illustrating the importance of interdisciplinary efforts in addressing complex environmental challenges. As industries look to adopt more sustainable practices, this development signifies a critical step in aligning chemical production with environmental sustainability goals, ensuring that future needs can be met without compromising the health of our planet.
The advancements showcased by LLNL researchers in the field of electrochemical processes present an optimistic outlook for the future of chemical manufacturing. As the industry grapples with the dual challenge of efficiency and environmental responsibility, this novel use of thin film technology for biomass conversion not only holds the promise of cleaner production but also lays the groundwork for broader shifts towards sustainability in chemistry. As researchers continue to refine these methods, the potential for a transformative impact on global chemical practices becomes increasingly tangible.

Leave a Reply