Recent advancements in electrochemical separation technologies have ushered in a new era for sustainable chemical processing. Researchers at the University of Illinois Urbana-Champaign have pioneered a groundbreaking method using a specially engineered polymer that exhibits selective attraction to specific substances only when electrically activated. This innovative approach, highlighted in the journal JACS Au, functions through a mechanism called halogen bonding, marking a significant step forward in the realm of chemical separation techniques.
The traditional methods of chemical separation, which often lean heavily on energy-demanding processes like thermal methods or membrane filtration, are not only costly but also generate considerable waste. These standard methods typically lack precision, leading to significant inefficiencies in both time and resources. The recent research focuses on a more eco-friendly alternative that utilizes electrochemical methods, thereby promising reduced material waste and a sustainable energy source.
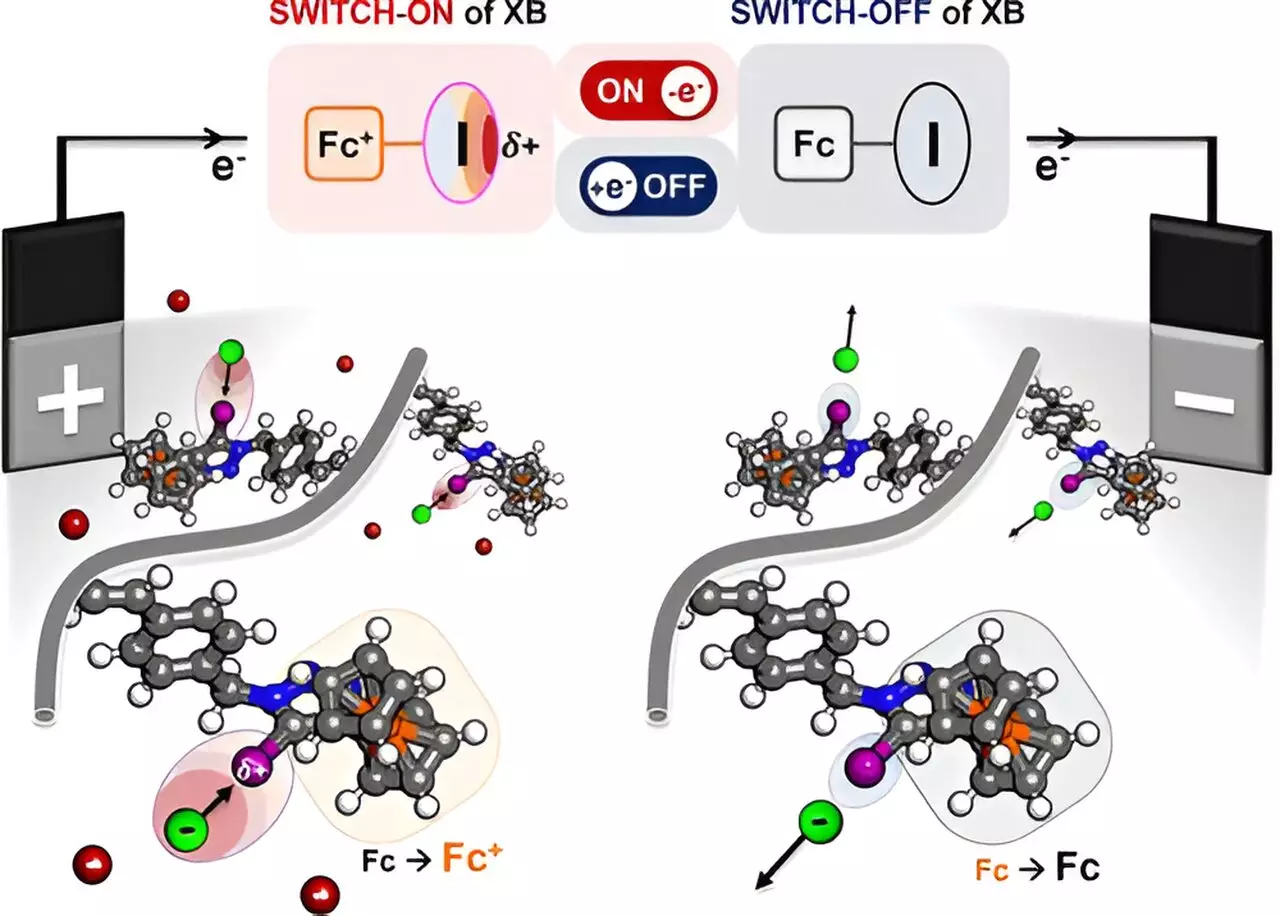
The crux of the innovation lies in the polymer’s ability to modulate the charge density on a halogen atom when electrified. Through this modulation, the polymer can selectively attract target molecules such as halides and organic compounds from mixed solutions. Professor Xiao Su, who spearheaded the project, poignantly compared the function of this polymer to a sponge engineered to absorb only the desired chemical from a mixture. This refined specificity enhances the overall efficiency of chemical processes, particularly in fields like pharmaceuticals and chemical synthesis, where precise separations can lead to enhanced yield and purity of products.
Central to the mechanism is the phenomenon of halogen bonding, wherein the polymer developed by Su and his team leverages a redox-responsive halogen donor. The halogen atom, specifically iodine, plays an essential role by creating a localized positive charge upon being electrified. This charge attracts negatively charged ions with substantial affinity, thus facilitating a selective pickup from a complex mixture.
The transformational capabilities of this polymer were validated through extensive experimental work. Following the polymer’s design, the researchers conducted tests in various organic solutions, successfully demonstrating the selective ion preference and confirming the presence of halogen bonding using advanced techniques like nuclear magnetic resonance and Raman scattering. This key verification lays the groundwork for further experimentation and application.
Moreover, the research was substantially bolstered through collaborative efforts with chemical and biomolecular engineering Professor Alex Mironenko, who delved into the computational aspects of the polymer. His contributions were crucial for unpacking the mechanisms that enable the redox center’s activation, enriching the study with insights that bridge theoretical underpinnings and practical applications. Such multidisciplinary collaboration is crucial for innovative advances in modern chemical engineering.
The implications of this new technology are vast. With the proof-of-concept established, the next phase revolves around refining the technique for real-world applications. Professor Su emphasized the importance of scaling strategies, potentially involving cascading models to enhance the separation purity further. There is a robust need to design continuous electrosorption systems capable of operating efficiently outside laboratory conditions, thereby translating this scientific innovation into practical solutions for industry-level challenges.
As the field stands on the precipice of transformation, harnessing electric-powered systems could lead to minimally invasive, highly efficient methods of chemical separation in various industries, which would be a significant stride towards sustainable practices. With ongoing refinement and exploration, this approach holds promise not just in improving separation efficacy, but also in advancing environmental sustainability and resource efficiency within the chemical engineering landscape.
The development of this selective electrochemical separation polymer exemplifies a pivotal advancement in sustainable chemical processes. By addressing the significant challenges posed by traditional separation techniques, this research not only enhances efficiency but also aligns with the growing demands for greener methodologies in chemical engineering. As further refinements unfold, the integration of such systems could redefine industrial practices, paving the way toward more sustainable and efficient chemical manufacturing. The future, indeed, looks brighter with innovations that are not just reactive but also proactive in addressing the pressing challenges of our time.

Leave a Reply