The process of reducing esters to produce desirable chemicals has long been associated with high costs, both financially and environmentally. Traditional methods often require the use of highly reactive and difficult to handle metal reductants, leading to challenges in terms of sustainability. However, a recent study conducted by researchers at the National Institutes of Natural Sciences (NINS) in Japan has introduced a novel approach to ester reduction using light as an energy source.
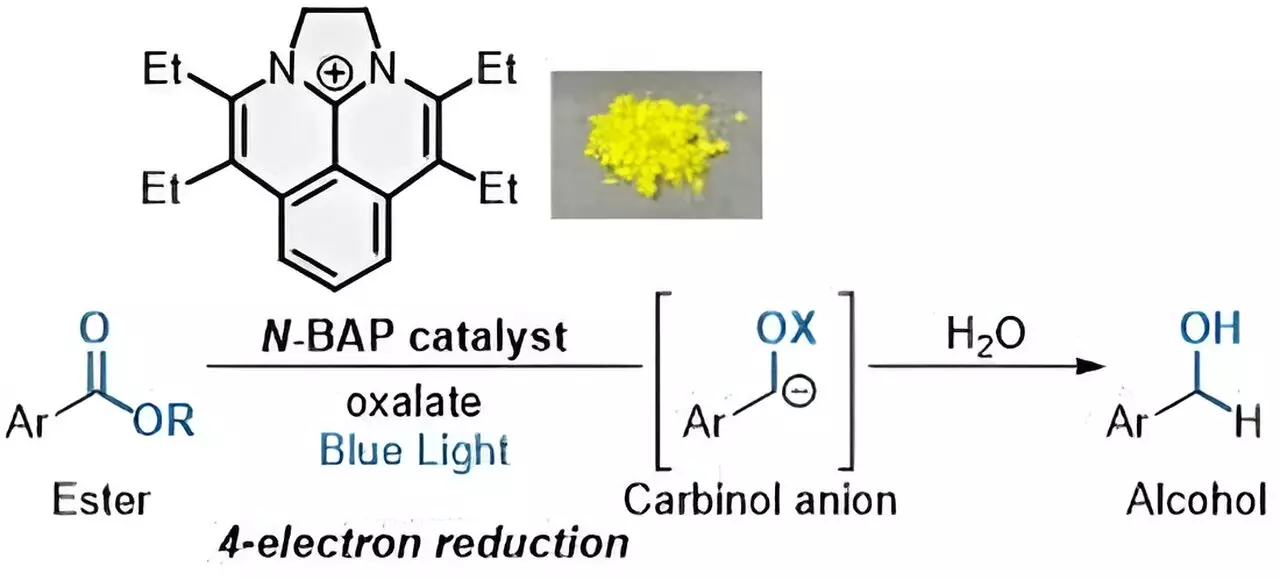
In a groundbreaking move, the researchers discovered that by utilizing sustainable photocatalysts, it is possible to facilitate the reduction of esters without the need for highly reactive metal reductants. Unlike conventional photocatalysts that rely on expensive and non-renewable noble metals, these sustainable catalysts activate when excited by light, promoting an electron transfer process between the catalyst and organic compounds. This method not only eliminates the use of reactive metal reductants but also enables the reduction of organic compounds by adding multiple electrons.
Challenges and Breakthroughs in Ester Reduction
One of the key challenges in ester reduction is the conversion of esters into alcohols, a process that requires the addition of four electrons. This complex multielectron transfer process has often posed difficulties for researchers in the past. However, the development of a new photocatalyst named “N-BAP” has paved the way for a rapid and sequential four-electron reduction of esters to generate carbinol anions, ultimately resulting in the formation of alcohols.
The use of photocatalytic reactions in organic synthesis has gained significant attention in recent years due to its alignment with the United Nations’ Sustainable Development Goals (SDGs). By harnessing visible light as an energy source and eliminating the need for reactive metal reductants, photocatalysts offer a more sustainable and environmentally friendly approach to chemical synthesis. The development of photocatalysts that can facilitate multielectron transfer processes represents a significant step forward in the field of organic chemistry.
The use of photocatalysts in ester reduction presents a promising avenue for achieving sustainable and cost-effective chemical synthesis. Through the innovative approach developed by the researchers at NINS, it is now possible to reduce esters to form alcohols using light as an energy source, thus minimizing the environmental impact of traditional chemical processes. The future of organic synthesis lies in the continued exploration and development of sustainable catalytic methods, paving the way for a more environmentally conscious approach to chemical production.

Leave a Reply