Samarium (Sm), classified as a rare earth metal, holds a crucial position in the field of organic chemistry primarily due to its ability to facilitate single-electron transfer reductions through its divalent compounds. The compound samarium iodide (SmI2) has demonstrated considerable stability, operating efficiently at room temperatures under mild conditions. This characteristic makes it particularly valuable in synthesizing pharmaceuticals and other biologically active compounds. However, the conventional usage of SmI2 poses significant challenges as it typically requires stoichiometric quantities of the reagent in reactions, alongside the involvement of hazardous chemicals. This often leads to processes that are both resource-dependent and costly to maintain, highlighting a pressing need for improvements in the methodology involving samarium.
Challenges in Reductive Transformations
Despite ongoing advances in synthetic methodologies employing samarium reagents, the majority of available techniques necessitate not only high quantities of samarium – often totaling 10% to 20% of the raw materials – but also the use of aggressive and highly reactive reducing agents. These factors constrain the practicality of the methods, particularly when coupled with the expense associated with samarium procurement. Therefore, the quest for a more efficient catalytic system that can minimize samarium usage while operating under benign conditions is a topic of burgeoning interest within the research community.
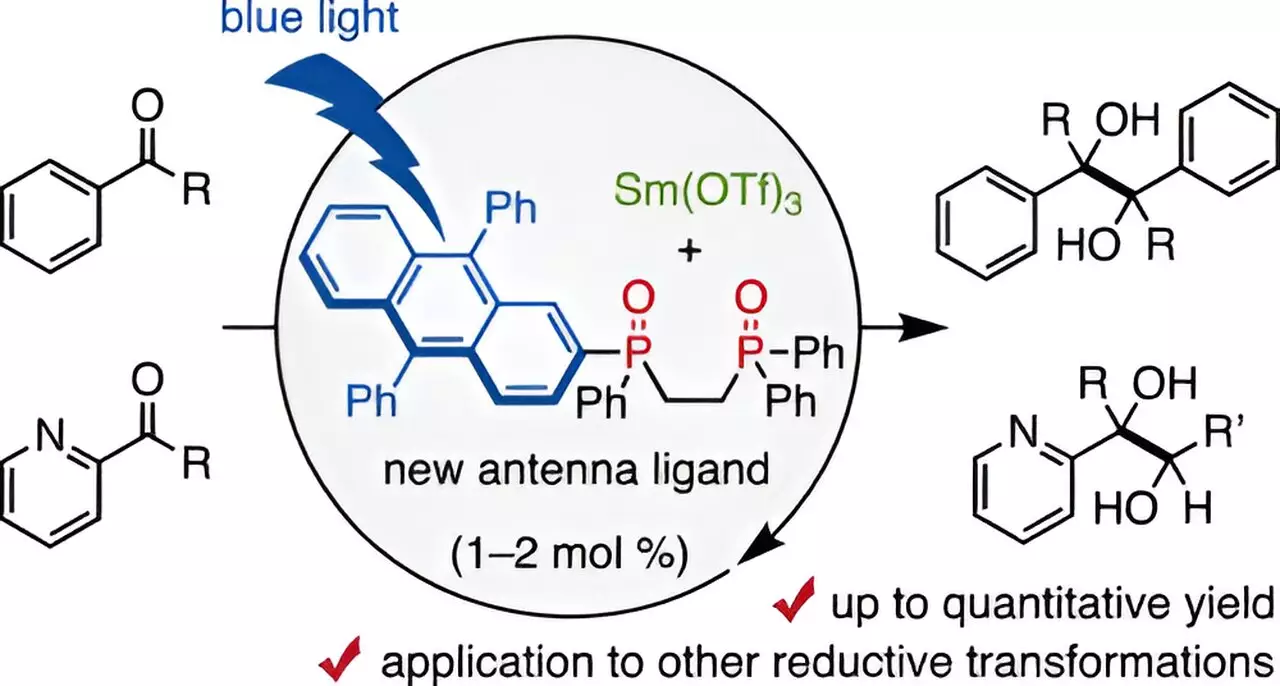
In a groundbreaking study led by Assistant Professor Takahito Kuribara and his team at Chiba University, a new approach has emerged that significantly reduces the amount of samarium needed in catalysis. Their innovative research focuses on the development of a novel ligand: a 9,10-diphenyl anthracene (DPA)-substituted bidentate phosphine oxide. This ligand acts as a “visible-light antenna,” optimally facilitating coordination with trivalent samarium, thereby enabling catalysis utilizing visible light as an energy source. This study, published in the Journal of the American Chemical Society, represents a notable advancement in the field, offering an efficient method to conduct reductive transformations with minimal samarium load.
The Mechanism of Action
The mechanism behind this innovative technique revolves around the structural design of the DPA-substituted bidentate phosphine oxide ligand, referred to as DPA-1. By employing blue-light irradiation, the resultant combination produces remarkable yields – as high as 98% in pinacol coupling reactions involving aldehydes and ketones, substances commonly integral to pharmaceutical synthesis. This efficiency is particularly impressive considering that only 1-2 mol% of the samarium catalyst is required, a stark decrease from the quantities standardly utilized in traditional methodologies.
While the research identified that the reaction could progress using mild organic reducing agents like amines, it also uncovered intriguing insights concerning the role of water in the reactions. It was noted that minimal water increased yields, while excess water acted counterproductively. This nuanced understanding encourages further experimentation in optimizing the reaction conditions for better outcomes.
To fully grasp the impressive performance of DPA-1, the researchers undertook an analysis of the emission properties of the samarium catalyst when paired with this ligand. The findings underscored that the DPA-1 acts not just as a passive coordinating agent, but as an active participant in the process, efficiently absorbing blue light and facilitating electron transfer to the samarium atom. Furthermore, the research demonstrated that the combination of the DPA-1 ligand with samarium could be extended to numerous molecular transformations, broadening the horizons of its applicative potential to carbon-carbon bond formations and the cleaving of carbon-oxygen and carbon-carbon bonds – all pivotal reactions in drug discovery and development.
Assistant Professor Kuribara encapsulated the significance of this advancement by stating, “Our new visible-light antenna ligand reduced the amount of Sm to 1-2 mol%, a significant decrease compared to stoichiometric amounts, by harnessing low-energy visible light.” The study emphasized a positive shift in utilizing trivalent samarium, which is more stable and manageable than its divalent counterpart.
This research not only unlocks novel avenues for samarium-based catalytic systems but also sets a precedent for future investigations. It illuminates potential pathways to develop efficient max-load and low-risk methodologies in organic chemistry, driving forward sustainable practices in the synthesis of complex chemical compounds. As this innovative method garners attention, it becomes a catalyst for change, heralding an era of efficient organic synthesis powered by visible light and minimal reagents.

Leave a Reply