In a groundbreaking study from the University of Freiburg, researchers, led by Professor Ingo Krossing, have revealed a significant advancement in the realm of molecular and coordination chemistry. This exploration not only broadens our understanding of oxidation potentials but also opens the gateway for innovative applications in electrocatalysis and beyond. Before delving deeper into this monumental finding, it is essential to contextualize the significance of the oxidation potential of positive ions, commonly utilized in diverse chemical applications.
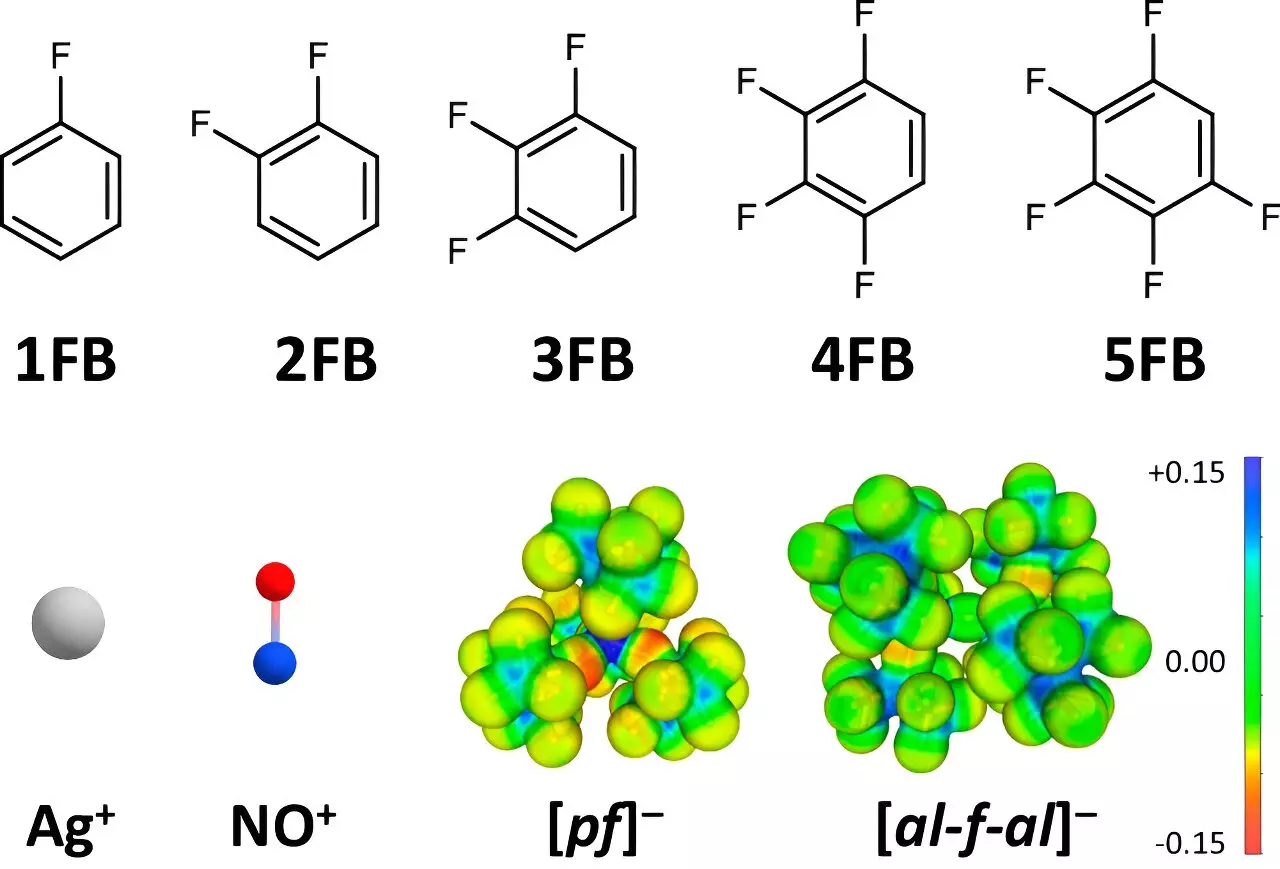
Traditional methods for generating oxidation potentials in chemistry have typically relied on conventional solvents and ions, capping the accessible potentials of common positive ions like Ag+ and NO+ at around +0.65 to +1.0 V relative to the ferrocene/ferrocenium couple (Fc+/0). These ions are prime examples of oxidizing agents that have been widely used in chemistry and materials science for their ability to effectively extract electrons from substrates. However, their interaction with solvents and anions often undermines their oxidation potential, acting as a limiting factor in various chemical reactions.
Krossing and his team undertook an ambitious approach to expand the oxidation capabilities of these positive ions. By employing weakly coordinating anions (WCAs) and strategically chosen solvents, particularly fluorinated benzene derivatives, they effectively increased the oxidation potential to impressive levels of +1.50 to +1.52 V. This significant advancement is spearheaded by a clever manipulation of solvent properties, notably the dielectric constants of the solvents utilized.
In collaboration with Dr. Johannes Hunger from the Max Planck Institute for Polymer Research, the research team discovered that the dielectric constants of these fluorinated solvents were markedly superior to those of conventional solvents such as acetone or dichloromethane. This enhancement of dielectric properties allows for a more effective stabilization of charged species, minimizing the adverse influences of solvent interactions.
To further substantiate their findings, the authors employed single-crystal X-ray diffraction techniques to analyze the solid-state structures of the complexes formed between fluorinated benzene derivatives and the positive ions. This meticulous investigation revealed a profound insight: the degree of fluorination inversely correlates with ion-solvent interactions. Essentially, as the number of fluorine atoms increases, the disruptive influence of the solvent on these positive ions diminishes significantly.
Co-author Dr. Malte Sellin articulated the essence of this structural exploration, noting that the ability to maintain nearly untouched oxidation states in these particles paves the way for exploratory research that was previously thought to be unattainable in chemical studies. The implications of this finding are substantial, as it heralds a new era for investigations into chemical reactivity and catalysis.
The implications of the team’s findings extend far beyond just theoretical interest. With the elevated oxidation potentials showcased by these fluorinated solvents, numerous applications emerge, particularly in the fields of electrocatalysis and redox processes, including redox shuttles and mediators. The ability to invoke oxidation in traditionally resistant substrates opens avenues for novel synthetic pathways, which could revolutionize chemical manufacturing and material science.
Moreover, this research may fundamentally alter the landscape for environmental applications, where more efficient oxidation processes could lead to improved methods for pollutant remediation. It presents not merely a technical advancement but an opportunity for chemists to rethink their strategies for designing redox-active systems.
The strategic advancements in oxidation potentials demonstrated by Professor Krossing and his team underscore the importance of molecular design in chemistry. Their innovative methodologies expand our horizons, inviting further inquiries and explorations into the intricate world of coordination chemistry. As the field continues to evolve, the possibilities born from this research promise a remarkable transformation in both academic and practical realms of chemistry.

Leave a Reply