In today’s world, polypropylene has secured its place as an integral component in numerous products that we rely on daily, from food storage solutions to vital medical gear. This surge in demand for polypropylene translates directly into an increasing need for its primary building block: propylene. Traditionally, propylene has been manufactured via complex processes that often rely on precious metals and high energy consumption, making it an expensive and environmentally taxing endeavor. However, recent breakthroughs in catalytic conversion processes offer a promising route toward more efficient, cost-effective production of propylene.
Breaking New Ground in Catalysis
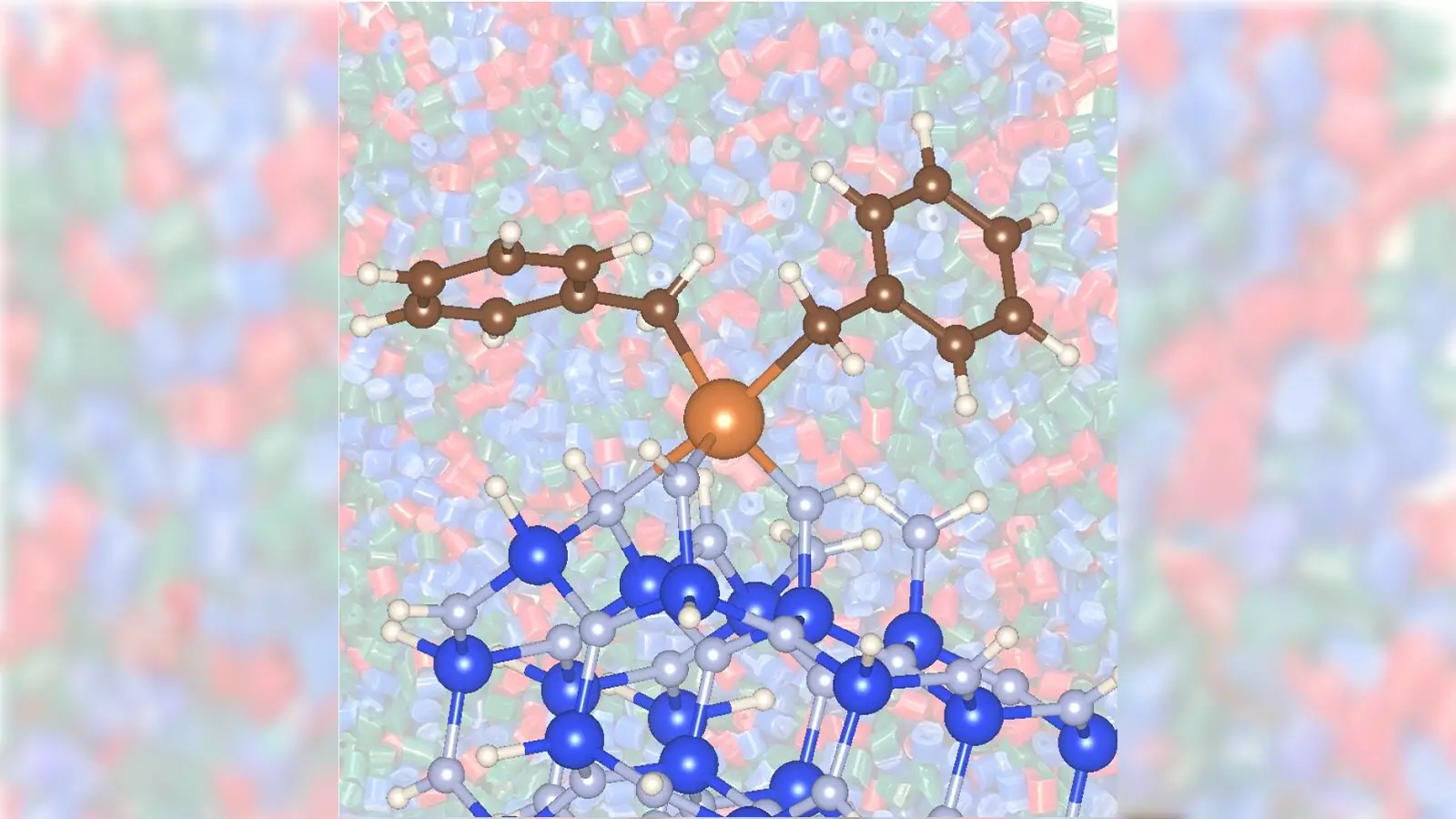
A collaborative project between scientists at the U.S. Department of Energy’s Argonne National Laboratory and Ames National Laboratory points to a groundbreaking advancement in the catalytic conversion of propane into propylene. By utilizing a zirconium catalyst paired with silicon nitride support, the researchers have developed a method that dramatically enhances the efficiency and speed of propylene production. With these innovations, the traditional reliance on costly platinum or toxic chromium catalysts may soon become a relic of the past.
The transition from propane to propylene has, historically, required a catalyst to speed up the reaction. However, conventional catalysts come with high operational temperatures and substantial energy use, leading to costly production practices and elevated carbon emissions. The newly discovered combination of zirconium and silicon nitride represents a paradigm shift that addresses these issues head-on.
Energy Efficiency and Environmental Impact
One of the standout qualities of this new catalysis process is its ability to operate at significantly lower temperatures—specifically, 842 degrees Fahrenheit compared to the typical 1,022 degrees. Lower temperatures not only reduce energy consumption but also limit the overall carbon dioxide emissions linked to this stage of plastic production. Given that carbon dioxide represents almost 80% of greenhouse gas emissions in the U.S., even marginal reductions in emissions can have substantial beneficial effects on climate change and air quality.
Moreover, this research illustrates an exciting opportunity to explore inexpensive yet effective non-precious metals that can emulate these catalytic properties. Improving production efficiency in this manner can lead to a reduction in overall consumer prices for products that rely on polypropylene, potentially making sustainable choices more accessible to the average consumer.
The Role of Catalyst Support Materials
The study illustrated the essential role that catalyst support materials, such as silicon nitride, play in promoting catalysis. In experiments, the zirconium catalyst demonstrated significantly improved activity when supported by silicon nitride compared to traditional silica support. This not only enhances the catalytic process but also emphasizes a critical area in which innovation can flourish—the use of unconventional metal catalysts on non-traditional support materials.
The findings suggest that expanding the repertoire of materials used in catalytic production can lead to superior results, opening doors to further advancements in chemical engineering and manufacturing processes. Such innovations are not only beneficial for existing methods but also pave the way for new discoveries in catalysis, potentially revolutionizing the field.
Scientific Collaboration: A Catalyst for Innovation
The successful outcomes of this research can be attributed, in part, to the rich collaborative environment fostered at Argonne and Ames. Leading scientists David Kaphan and Max Delferro expressed excitement over the interdisciplinary nature of their work, illustrating how pooling various specializations can yield results that would be unattainable in isolated research. By leveraging advanced analytical techniques like X-ray absorption spectroscopy and dynamic nuclear polarization-enhanced nuclear magnetic resonance, they gained a comprehensive understanding of the interactions at play between catalysts and support materials.
The research community’s commitment to teamwork emphasizes that innovation in science often emerges from collaborative endeavors. This collective approach not only enhances the individual strengths of each team but also creates a fertile atmosphere for groundbreaking discoveries that can reshape industrial practices for the better.
As the world grapples with the dual challenges of heightened consumer demand and the necessity for sustainable practices, the advancements in the catalytic conversion of propane to propylene emerge as a beacon of change. The use of zirconium and silicon nitride not only streamlines production but also provides a glimpse into a future where chemical manufacturing is both eco-friendly and economically feasible. This paradigm shift holds promise for a more sustainable planet, where technological innovations contribute to a circular economy rather than deplete it. It’s initiatives like these that signal a brighter tomorrow, demonstrating that intelligent science can lead to substantial progress in how we approach the very materials that compose our daily lives.

Leave a Reply