The escalating issues of water pollution have necessitated advanced scientific inquiries to develop effective remediation technologies. A recent study conducted by a collaborative team from the University of Science and Technology of China (USTC) and the Suzhou Institute for Advanced Study has revealed a groundbreaking approach leveraging single-atom catalysts (SACs) within a Fenton-like catalytic framework. The implications of this work, documented in *Nature Communications*, hold promise for transforming how pollutants can be addressed in aquatic environments.
Water contamination is primarily driven by various harmful pollutants, including pesticides, heavy metals, and organic compounds. Traditional methods of purification often suffer from significant limitations due to inadequate reaction speeds and inefficient oxidant usage. Previous innovations utilized nanoconfined SACs; however, the mechanisms underlying their enhanced efficiency had remained elusive. The research team demonstrated that the primary hurdles stemmed from the inadequate transport of reactants and the excessive oxidant requirements in these processes.
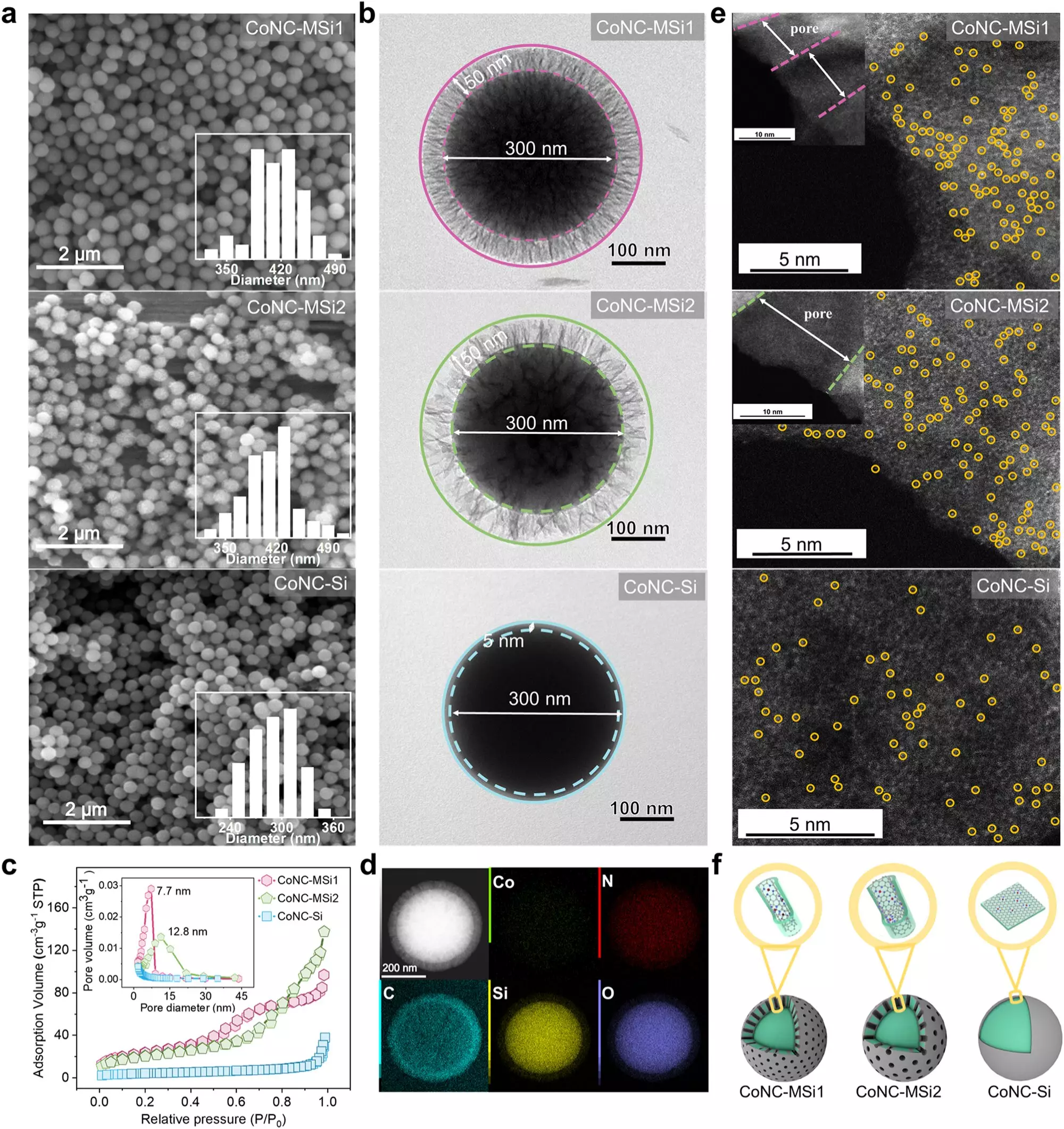
In a quest for optimization, researchers introduced a method whereby SACs were confined within microscopically tiny pores in silica matrices. This innovative confinement strategy enabled a remarkable acceleration in reaction rates. More substantially, the study found a paradigm shift in the catalytic pathways; rather than depending on the highly reactive singlet oxygen for chemical degradation, the process transitioned to direct electron transfer mechanisms. This fundamental shift is critical—it drastically increases the effectiveness of pollutant breakdown, achieving a staggering 34.7-fold increase in degradation rates compared to conventional techniques.
Not only did the study present a leap in reaction kinetics, but it also addressed the sustainability of oxidant usage. The researchers quantified a striking enhancement in oxidant efficiency—from an original 61.8% to an astonishing 96.6%—a significant advancement that could reduce the costs and environmental impacts associated with water treatment technologies. Such efficiency is pivotal in ensuring that methods of purification can be both effective and sustainable in real-world applications.
The success of the SAC/pores system was corroborated through various tests, particularly in degrading phenolic compounds known for their electron-rich characteristics. The robustness of this method across different environmental conditions and its effectiveness in real lake water suggest a wide range of applications in diverse settings. This successful employment of SACs confirms their potential utility in developing advanced oxidation processes and further bolsters the pursuit of low-carbon technologies for environmental remediation.
The findings from this research represent a pivotal advancement in the field of water purification. By providing a clearer understanding of the mechanisms at play in nanoconfined catalysts, the study opens doors to future innovations aimed at enhancing pollution control. As the global community grapples with escalating water quality issues, embracing such technologies could lead to effective solutions for a cleaner, healthier planet. The work highlights the importance of interdisciplinary collaboration in tackling environmental challenges—an essential stride toward sustainability in water treatment practices.

Leave a Reply