Basic oxide catalysts play a significant role in the synthesis of chemicals, pharmaceuticals, and petrochemicals. These catalysts contain oxygen ions with unpaired electrons that can be shared with other species to facilitate chemical reactions. In recent years, there has been a growing interest in improving the catalytic power of basic oxide catalysts by enhancing their basicity, which refers to their ability to donate electrons or accept hydrogen ions.
Scientists and researchers have implemented various strategies to enhance the basicity of basic oxide catalysts. Some of these strategies include doping the catalyst with highly electronegative cations like alkali metals, substituting oxide ions with anions of different valences such as hydride (H-) or nitride (N3-) ions, and increasing the electron density in the catalyst by introducing oxygen vacancies next to oxide anions.
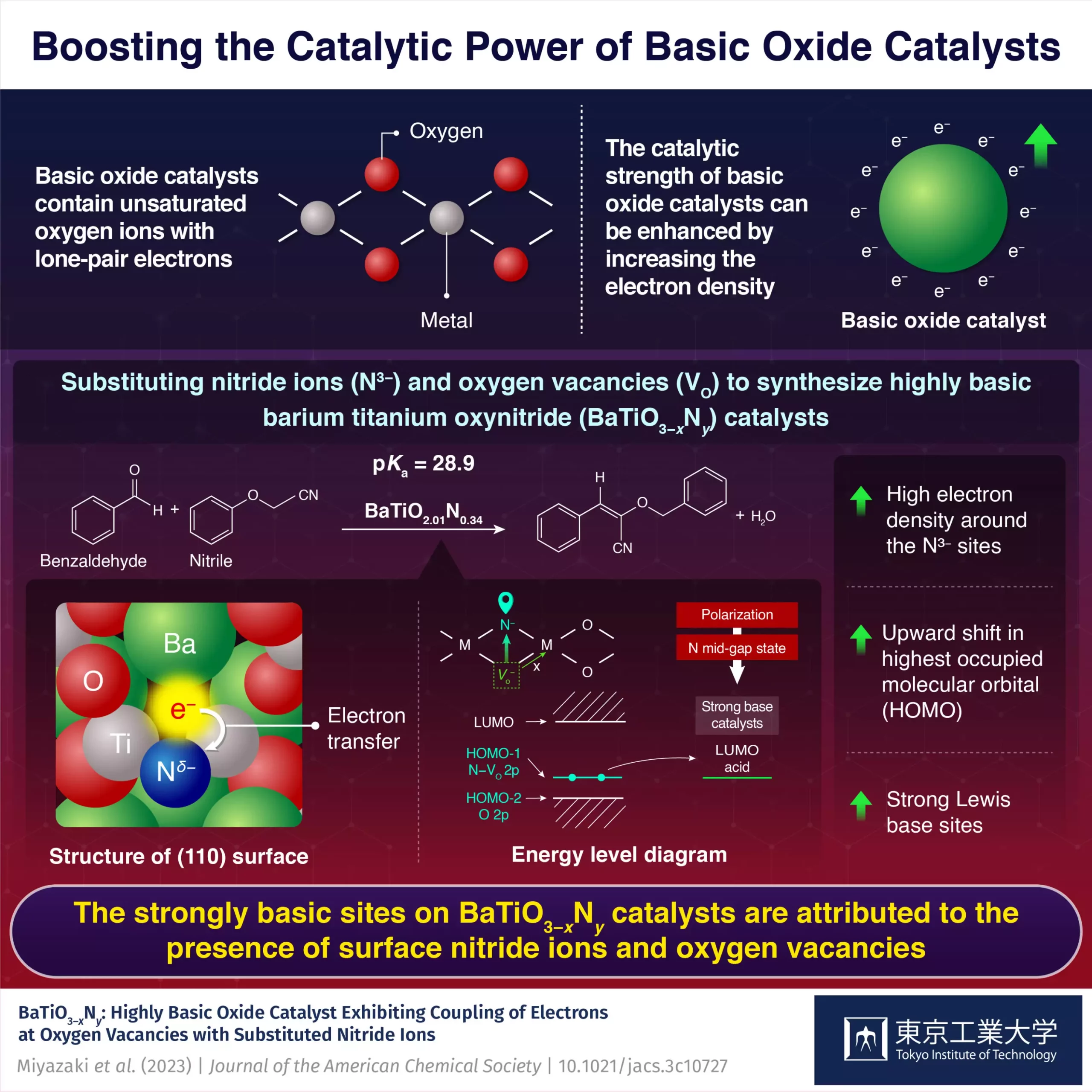
In a groundbreaking study conducted by a team of researchers from the Tokyo Institute of Technology, a hexagonal BaTiO3–xNy oxynitride catalyst with basicity comparable to that of superbases was developed. By substituting nitride ions and oxygen vacancies into face-sharing Ti2O9 dimer sites in BaTiO3−x, the researchers achieved remarkable results.
The substitution of oxygen ions with nitride ions led to a significant change in the electronic structure of the catalyst, resulting in an upward shift of the energy level of the highest occupied molecular orbitals (HOMO). This shift made it more favorable for electrons to be donated to a reactant’s lowest unoccupied molecular orbital (LUMO). Additionally, the introduction of oxygen vacancies adjacent to the doped nitride ions increased the electron density, further raising the HOMO energy level and creating a highly basic catalyst with a strong tendency to donate electrons.
The coupling of substituted nitride ions to electrons at oxygen vacancies is the key factor contributing to the improved basicity of the oxynitride catalyst. This unique synergy between the nitride ions and oxygen vacancies enhances the basicity and facilitates Knoevenagel condensation reactions.
The highly basic nature of the oxynitride catalyst proved to be advantageous in Knoevenagel condensation reactions. The catalyst accepted protons from highly basic nitrile reactants with pKa values as high as 23.8 and 28.9, indicating a basic strength comparable to that of superbases. Furthermore, the oxynitride catalyst demonstrated exceptional stability, maintaining its structure and electronic state even after repeated use.
The development of highly basic catalysts has significant implications for various chemical processes. The success of this study in improving the basicity of basic oxide catalysts opens up possibilities for the synthesis of more highly basic catalysts by combining surface anion species and vacancies. This breakthrough in catalytic design has the potential to revolutionize the field of chemistry and pave the way for more efficient and sustainable chemical reactions.
The development of the hexagonal BaTiO3–xNy oxynitride catalyst represents a remarkable advancement in the quest for highly basic catalysts. Through innovative structural modifications, the researchers achieved a catalyst with comparable basicity to superbases, demonstrating its potential for practical applications. The synergistic effect of nitride ions and oxygen vacancies played a crucial role in enhancing the basicity and stability of the catalyst. Moving forward, further research and exploration of surface anion species and vacancies hold the key to the synthesis of even more highly basic catalysts with transformative capabilities in the field of chemistry.

Leave a Reply