Photocatalysts that are highly reducing or oxidizing are a major challenge in the field of photochemistry. Up until now, only a small number of transition metal complexes, specifically those with Earth-abundant metal ions like chromium, iron, and cobalt, have been successful in becoming excited state oxidants. However, these photocatalysts require high energy light for excitation and their oxidizing power has not been fully exploited. Additionally, most of these catalysts rely on precious and expensive metals. This is why the recent development of a new molecular system based on the element manganese is generating significant interest among researchers. Manganese is the third most abundant metal, after iron and titanium, and is therefore widely available and inexpensive. This article will delve into the details of this new breakthrough in photochemistry and its potential impact on the field.
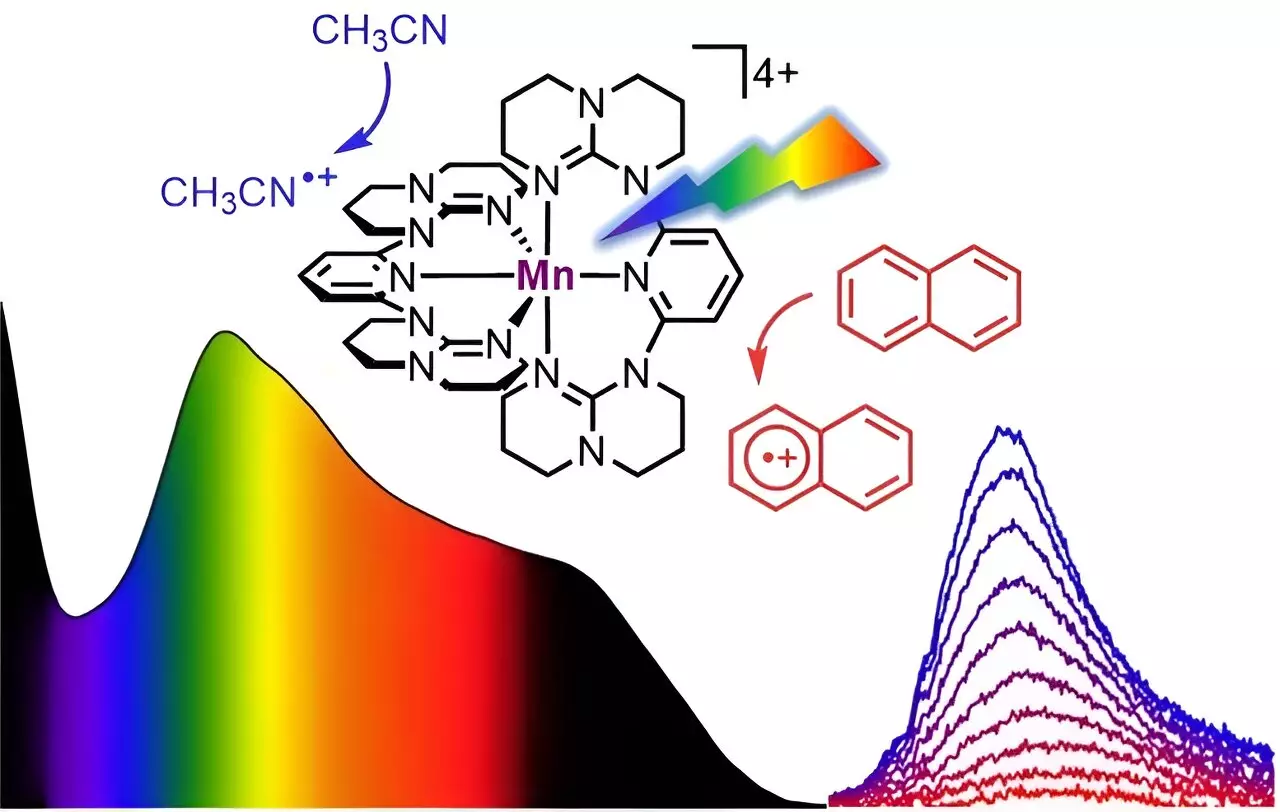
A team of researchers, led by Professor Katja Heinze of Johannes Gutenberg University Mainz (JGU), has successfully designed a soluble manganese complex that exhibits panchromatic absorption. This means that the complex is able to absorb all visible light, from blue to red, as well as parts of the near-infrared spectrum. The absorption properties of the complex are reminiscent of the dark color of Braunstein or manganese dioxide, a naturally occurring mineral. Interestingly, unlike its mineral counterpart, the “molecular Braunstein” emits near-infrared light with a wavelength of 1,435 nanometers when excited with visible or near-infrared light at a wavelength of 850 nanometers.
One of the most exciting observations from this research is that the “molecular Braunstein” is capable of oxidizing various organic substrates after photoexcitation. This includes aromatic molecules with very high oxidation potentials such as naphthalene, toluene, and benzene. The oxidizing power of the complex is so strong that even stable solvents can be attacked when excited by LED light. This discovery opens up new possibilities for challenging light-driven reactions using manganese, which is a common and abundant metal.
Ultrafast spectroscopic techniques, which use laser pulses with sub-picosecond time resolution, revealed an unusual excited-state reactivity in the “molecular Braunstein” complex. The researchers identified two different photoactive states: a short-lived but highly oxidizing high-energy state and a longer-lived moderately oxidizing lower-energy state. The high-energy state can attack solvent molecules that are in close proximity before excitation, while the lower-energy state exists long enough to attack aromatic substrates after diffusional collision. This phenomenon is known as static and dynamic quenching of the excited states.
To gain a deeper understanding of the photoinduced processes involved in the manganese-based photocatalyst, the researchers used quantum chemical calculations in combination with the spectroscopic results. These calculations provided a detailed picture of the various excited states and their behavior. However, due to the complexity of the calculations, the researchers relied on the computing power of supercomputers such as MOGON and ELWETRITSCH in Rhineland-Palatinate.
The development of a highly efficient and cost-effective manganese-based photocatalyst has the potential to revolutionize the field of photochemistry. This new system has the capability to replace the rare and expensive ruthenium and iridium compounds, which are currently the most commonly used photocatalysts. Additionally, it opens up the possibility of exploring new reaction and substrate classes that were previously unavailable with traditional compounds. This advancement aligns with the growing need for sustainable photochemistry, and the researchers involved in this study are determined to continue pushing the boundaries of the field.
The discovery and development of a soluble manganese complex with panchromatic absorption and strong oxidizing power is a significant breakthrough in the field of photochemistry. By utilizing a common and abundant metal, this new photocatalyst has the potential to revolutionize light-driven reactions and enable the exploration of uncharted reaction and substrate classes. The unusual excited-state reactivity and the ability to oxidize various organic substrates make this development particularly promising. With further research and refinement, scientists can pave the way for a more sustainable and efficient future in photochemistry.

Leave a Reply