Chemical diversification of proteins plays a crucial role in understanding biological processes and the intricate structures of proteins. In a recent breakthrough, researchers from the Max Planck Society have published their groundbreaking findings on amino acid modification in Nature Chemistry. This article will delve into the novel technique developed by the research group and explore its implications in the field of chemical biology.
Traditionally, the modification of proteins has involved complex multistep syntheses, limiting the range of modifications that can be made on specific amino acids. This method often requires external reagents, which pose challenges for the introduction of linchpin modifications. Moreover, the use of external reagents may result in decreased reactivity, restricting the functionalization process. With these limitations in mind, the research group of Tobias Ritter sought to develop a more efficient and versatile technique for protein modification.
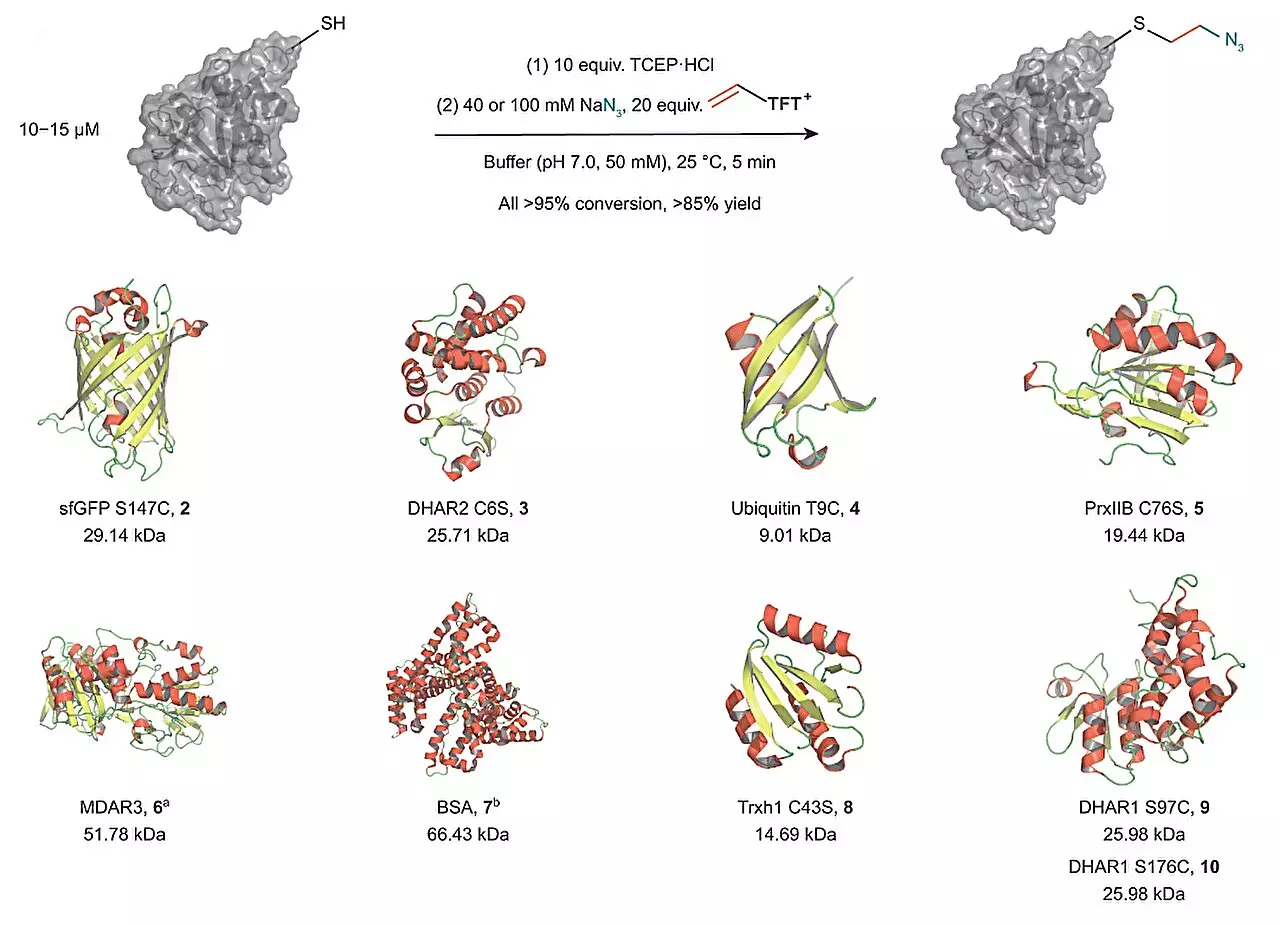
The Ritter group’s groundbreaking technique involves the utilization of vinyl thianthrenium salts to transform cysteine, a prominent amino acid, into a highly reactive episulfonium electrophile in situ. By using this one-pot process, the researchers were able to introduce a highly reactive intermediate that allows for the connection of cysteine with various external nucleophiles. This revolutionary approach eliminates the need for additional steps and enables the scientists to diversify the proteins rapidly.
The introduction of the highly reactive intermediate opens up a world of possibilities for protein modification. Not only does it allow for the easy connection of different biorelevant functional groups to proteins, but it also offers a stable ethylene linkage close to the protein’s surface. This method provides a new and attractive way to add labels or functionalities that alter the chemical environment of a protein.
Additionally, the reactivity of the episulfonium intermediate enables protein-protein ligation and macrocyclization of linear peptides. These techniques have profound implications in the study of protein complexes and their biological activity. Furthermore, these modifications enhance the stability of peptides against biological degradation, making them promising candidates for drug development.
The synthesis of vinyl thianthrenium salts from ethylene gas also allows for the production of reagents with different compositions of isotopes. Isotope-labeled compounds possess the same reactivity as their non-labeled counterparts but differ slightly in molecular weight. These compounds are highly valuable in state-of-the-art mass spectrometry proteomics research, as they enable the extraction of quantitative information from whole cellular systems.
The introduction of the novel technique utilizing vinyl thianthrenium salts marks a significant advancement in the field of chemical biology. This one-pot process enables the rapid diversification of proteins and facilitates the connection of different functional groups to proteins. It also allows for protein-protein ligation, macrocyclization of linear peptides, and enhanced stability against biological degradation. Additionally, the synthesis of isotope-labeled compounds using vinyl thianthrenium salts opens up avenues for quantitative analysis in mass spectrometry proteomics research. Overall, this technique presents a valuable and widely applicable tool for exploring the chemical diversification of proteins.

Leave a Reply