In the pursuit of a cleaner environment, the challenge of eliminating toxic nitrogen oxides from industrial emissions stands paramount. Among the various chemical solutions available, zeolites have emerged as effective catalysts. Their unique porous structure enables them to capture and convert harmful gases, such as nitric oxide (NO) and nitrous oxide (N2O), into benign substances. These nitrogen oxides are not just pollutants; they contribute to serious environmental issues like acid rain and global warming. Understanding how zeolite catalysts function at the molecular level is crucial for enhancing their efficacy and reducing industrial pollution.
A significant study carried out by researchers at the Paul Scherrer Institute (PSI), in partnership with the Swiss chemical company CASALE SA, sheds light on the dynamics of zeolite catalysts. The study, published in the journal Nature Catalysis, aimed to decipher the intricate mechanisms behind the activity of zeolite-based catalysts in eliminating nitrogen oxides. Davide Ferri, the research group’s leader, emphasizes the importance of this research, noting that such insights can inform the future development of more efficient catalytic systems. The collaborative nature of this research underscores the need for interdisciplinary approaches to tackle pressing environmental challenges.
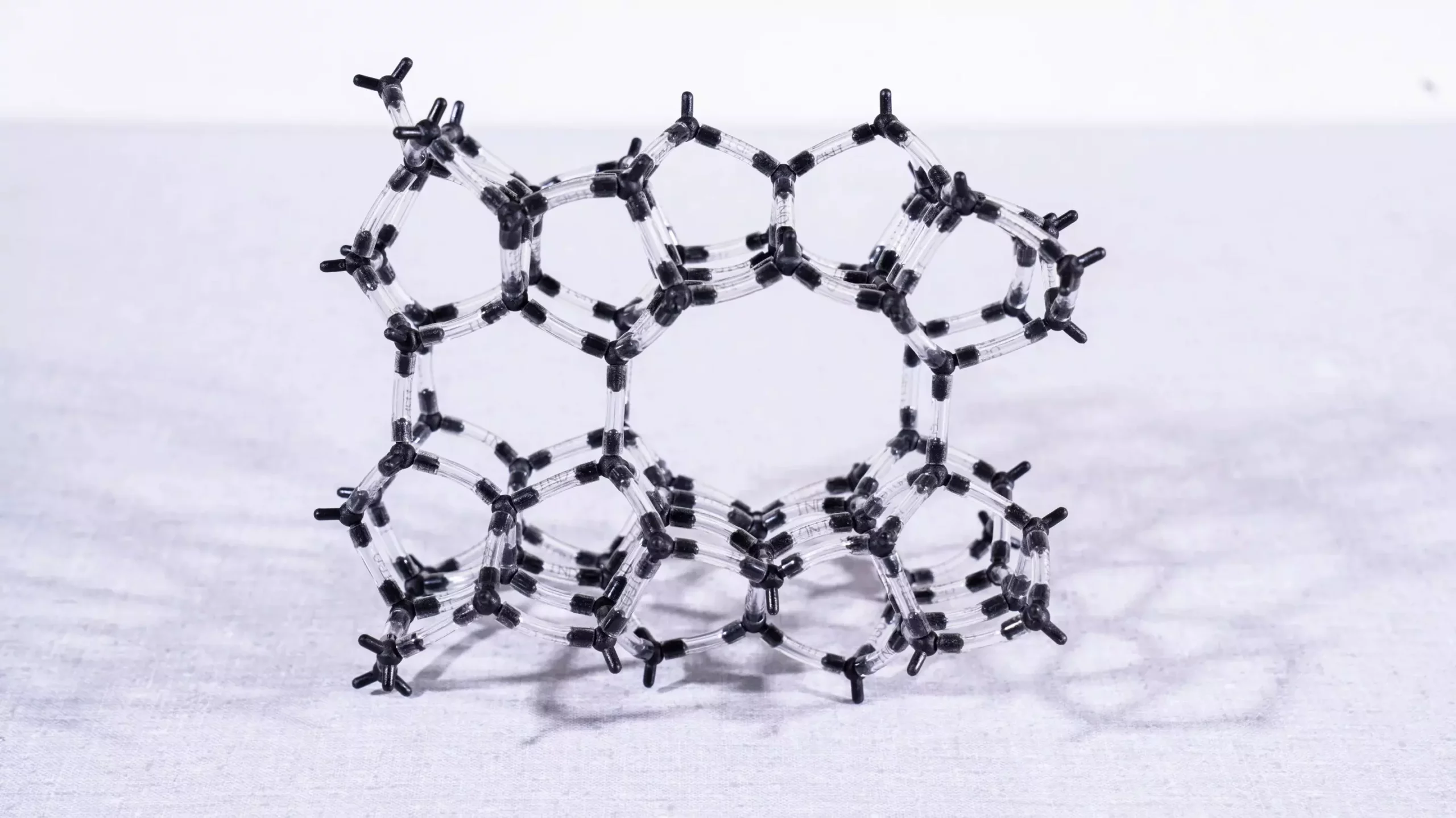
Decoding the Structure of Zeolite Catalysts
Zeolites, which can naturally occur or be synthesized, feature a complex framework composed primarily of aluminum, oxygen, and silicon. This structural configuration is crucial, as it forms a scaffold that houses various active elements, including iron. The placement and configuration of iron within the zeolite framework strongly influence its catalytic properties. According to Filippo Buttignol, one of the researchers involved in the study, iron can exist in numerous forms within the zeolite matrix—ranging from single atoms in small cavities to clusters of multiple atoms in larger voids. The challenge was to identify which specific forms of iron are effective in catalyzing the reactions that convert NO and N2O into harmless substances.
Employing Advanced Spectroscopy Techniques
To unravel the mystery behind the catalytic activity of iron species in zeolites, the research team implemented a series of sophisticated spectroscopic analyses. They first utilized the Swiss Light Source (SLS) at PSI to conduct X-ray absorption spectroscopy, allowing them to observe a wide array of iron species in real-time throughout the catalytic process. Subsequent evaluations included electron paramagnetic resonance spectroscopy, conducted in collaboration with ETH Zurich, and infrared spectroscopy, which helped delineate the molecular behaviors of the different iron species involved. Each of these methodologies contributed essential insights that culminated in a clearer understanding of the catalytic mechanism at play.
The findings of the research established that the catalytic activity primarily hinges on the interactions between individual iron atoms located in two strategically positioned sites within the zeolite structure. This precise arrangement facilitates a cooperative mechanism during the catalysis of nitrogen oxides. One iron atom, which specifically interacts with N2O, is situated at a center surrounded by a square of oxygen atoms. Meanwhile, the second iron atom, engaged in the reaction with NO, is encased in a tetrahedral arrangement of oxygen. This spatial relationship is critical; it ensures that the iron atoms can effectively transfer electrons in a cyclical redox process essential for catalysis. According to Buttignol, this cooperative behavior of iron atoms underlines the necessity for precise structural configurations to achieve optimal catalytic performance.
Implications for Industrial Applications
The implications of this research extend far beyond the scientific community; they hold significant promise for the industrial sector. With a more profound understanding of how zeolite catalysts operate, manufacturers can adjust their synthesis processes to refine the properties of these catalysts, enhancing their effectiveness in nitrogen oxide removal. As Ferri succinctly puts it, pinpointing the reaction sites opens pathways toward manufacturing better catalysts tailored for specific applications.
The urgent need for efficient methods to eliminate nitrogen oxides cannot be overstated. Both NO and N2O pose serious health risks as well as environmental concerns: nitric oxide contributes to the formation of acid rain, while nitrous oxide has a greenhouse effect almost 300 times more potent than carbon dioxide on a molecular level. Thus, advancing our understanding of zeolite catalysts signifies a critical step toward addressing the dual threats of pollution and climate change.
The research conducted by PSI and CASALE SA offers compelling insights into the functioning of zeolite catalysts in the removal of nitrogen oxides. By dissecting the roles of different iron species within the zeolite framework, scientists can not only contribute to a more sustainable industrial process but also lay the groundwork for enhanced environmental protection strategies. The journey toward greener industrial emissions continues, driven by innovative research and unwavering commitment to scientific excellence.

Leave a Reply