Water, an essential compound for life, is known for its unique properties that set it apart from other substances. One of the many peculiarities of water is the behavior of its molecules at the interface with air. While the majority of water molecules exhibit typical hydrogen bonding patterns, those at the surface experience different interactions. Understanding the dynamics of these surface molecules is crucial for deciphering the complexities of processes occurring at water surfaces.
Researchers at RIKEN have dedicated their efforts to unraveling the mysteries of surface water molecules. Despite extensive knowledge about the behavior of water molecules in the bulk liquid, there is a significant gap in understanding the dynamics at the interface. Tahei Tahara and his team have been at the forefront of developing advanced spectroscopy techniques to probe the interactions of surface water molecules, aiming to bridge this knowledge gap.
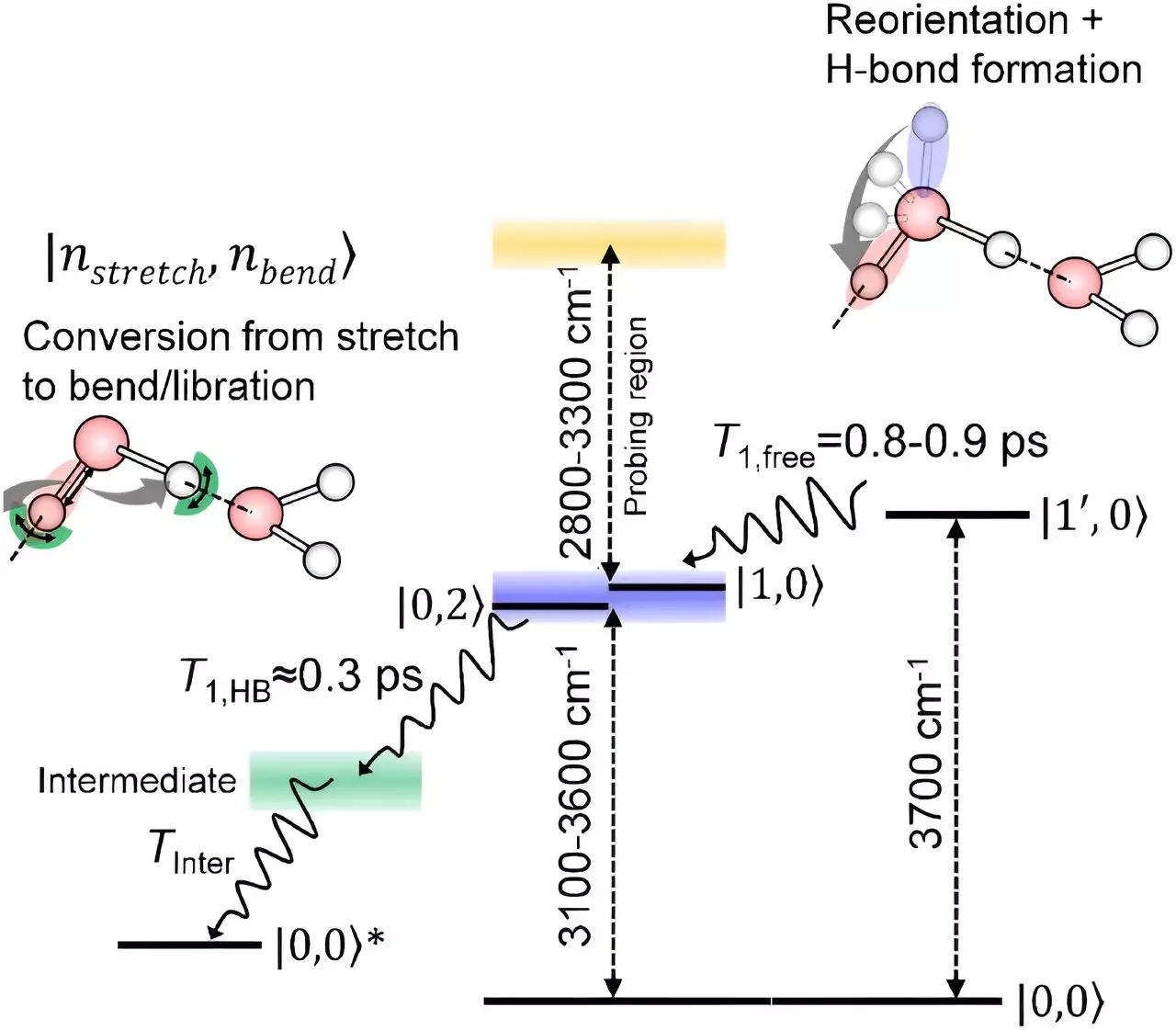
In a groundbreaking study published in Nature Communications, RIKEN scientists have shed light on how excited water molecules at the interface with air lose their energy. By employing a sophisticated technique based on infrared spectroscopy, the team was able to observe the relaxation of oxygen-hydrogen bonds in surface water molecules. This innovative approach unveiled a previously elusive aspect of the behavior of surface water molecules, providing a more comprehensive understanding of their dynamics.
The findings of the study revealed that oxygen-hydrogen bonds of surface water molecules exhibit a unique relaxation process. Initially, the bonds rotate without losing energy, followed by a relaxation pattern similar to that of molecules in the bulk liquid. Surprisingly, despite the differences in bonding at the surface, the relaxation process shares similarities with molecules inside the liquid. This discovery challenges previous assumptions about the behavior of surface water molecules and offers a detailed insight into the relaxation mechanisms at the interface.
The implications of this research extend beyond understanding the behavior of water molecules at the surface. Tahara and his team are now exploring the application of their spectroscopic technique to investigate chemical reactions occurring at the water-air interface. By delving deeper into the complexities of surface interactions, researchers can unlock a wealth of information that may have far-reaching implications in various scientific disciplines.
The study conducted by RIKEN scientists has significantly advanced our understanding of the dynamics of water molecules at the surface interface. Through innovative spectroscopy techniques and meticulous observations, the team has provided valuable insights into the relaxation processes of surface water molecules. This research not only enhances our comprehension of water’s anomalous properties but also paves the way for future investigations into surface interactions and chemical reactions.

Leave a Reply