The use of anodes for the electrolytic splitting of water is common in generating “green” hydrogen through electrolysis. However, the current state-of-the-art catalyst, iridium, lacks stability in the acidic environment of the electrolysis cell, leading to a decrease in its catalytic effect. To address this issue, a research team at the Helmholtz-Zentrum Berlin (HZB) and the Helmholtz Institute Erlangen-Nuremberg for Renewable Energies (HI-ERN) conducted a study on the addition of titanium oxide to iridium catalysts. The objective was to determine if varying proportions of titanium oxide can enhance the stability of the catalyst without compromising its catalytic activity.
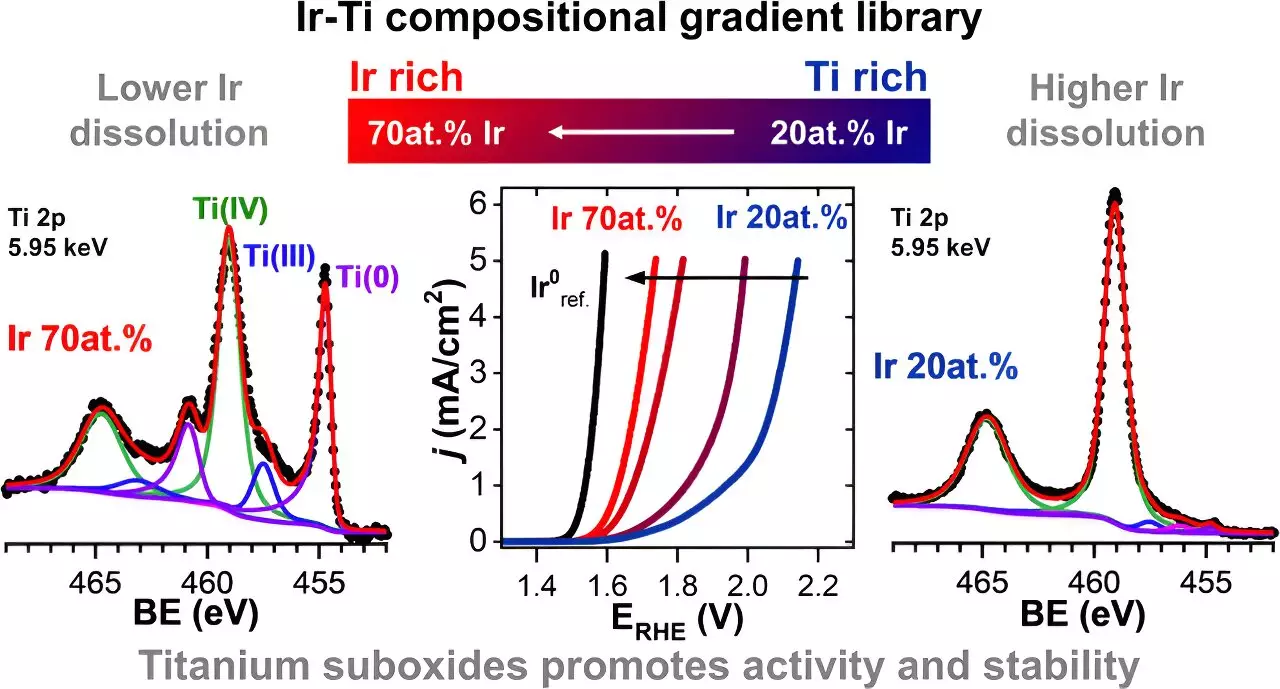
In order to investigate the effects of titanium oxide, a sample with systematically varied concentrations of iridium and titanium oxides was created. The team at HI-ERN, led by Prof Dr. Olga Kasian, produced a thin-film materials library using a sputtering technique to control the local compositions of titanium and iridium. The iridium content in the samples ranged from 20% to 70%.
Analyzing the Effects
To analyze the chemical structure of the mixed iridium-titanium oxide samples, the team utilized X-ray spectroscopic methods at the BESSY II synchrotron facility in the EMIL laboratory. The analysis revealed several interesting effects.
One significant finding was the improved conductivity of the material due to the presence of titanium suboxides, such as TiO and TiOx. This conductivity enhancement can contribute to more efficient electrolysis processes.
Another intriguing result was the faster dissolution of certain titanium oxides in the aqueous electrolyte compared to iridium. This phenomenon led to the formation of micropores on the surface, promoting the oxygen evolution reaction. Additionally, the dissolution of titanium oxides played a crucial role in reducing the dissolution of iridium itself. It was observed that the addition of titanium oxides, including TiO2, TiO, and TiOx, significantly decreased the dissolution of iridium. In fact, a sample with 30% titanium content exhibited an iridium dissolution rate approximately 70% lower than that of a pure iridium electrode material.
One might question the relevance of these laboratory findings for industrial applications. Prof Dr. Marcus Bär, who led the HZB team, acknowledges the challenges of implementing changes in established technologies. However, these research findings open up perspectives for improving the stability of iridium catalysts, which could have significant implications in the industrial production of “green” hydrogen.
The addition of titanium oxide to iridium catalysts has been shown to enhance their stability, addressing the issue of iridium dissolution in the acidic environment of electrolysis cells. The material library approach allowed for systematic exploration of different mixing ratios, with the team identifying the ideal proportion to reduce iridium dissolution. The study’s findings have important implications for future developments in the production of “green” hydrogen and highlight the potential for enhancing catalyst performance through the addition of stabilizing agents such as titanium oxide.

Leave a Reply