In the world of materials science, polymers play a crucial and versatile role, similar to how a train consists of multiple cars linked together to form a single unit. This analogy effectively encapsulates the relationship between monomers and polymers; while monomers serve as the foundational building blocks, polymers are the macromolecules formed through their chemical bonds. However, the cross-linking of monomers constrains the functionality of the resulting polymers, as they typically rely on a limited range of chemically identical building blocks. Recent advances by chemists at Scripps Research, alongside cooperation from prominent universities, have introduced groundbreaking methods to synthesize novel monomers in a controlled environment, potentially revolutionizing various applications, from drug delivery to advanced electronics.
A detailed study featured in *Nature Synthesis* discusses an innovative reaction that utilizes nickel as a catalyst to manipulate the structural properties of monomers. This approach allows scientists to create polymers that possess customizable properties tailored for specific functions. The lead researcher, Dr. Keary Engle, emphasizes that employing earth-abundant metals like nickel could catalyze the development of diverse materials previously unexplored. The ability to modify the chemical backbone of polymers opens a pathway for an array of applications, illuminating the vast potential within polymer chemistry.
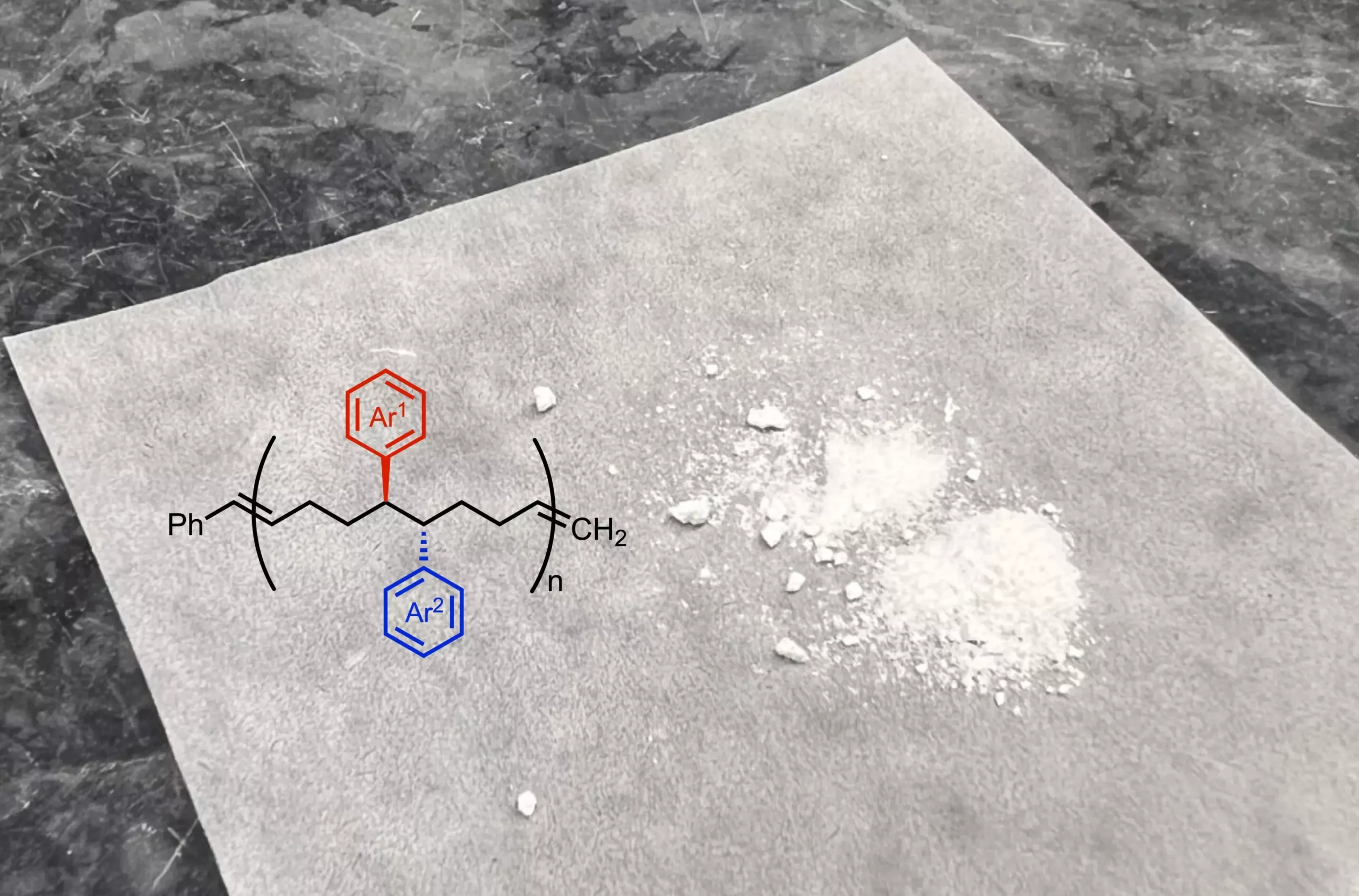
The collaborative effort involved polymer researchers from the Georgia Institute of Technology and the University of Pittsburgh, showcasing an interdisciplinary approach to tackling complex challenges in polymer synthesis. Dr. Anne Ravn, a co-first author of the study, stresses the correlation between the chemistry of the monomer building blocks and the resultant polymer characteristics. By adjusting the chemical structure of the building blocks, researchers can fundamentally alter the properties of the macromolecules created.
To produce these groundbreaking monomers, the research team pioneered a reaction that adds two functional groups to a starting molecule. These functional groups are integral in conferring distinct chemical and physical attributes to the resulting polymers—affecting properties such as flexibility, elasticity, and solubility. This methodological evolution allows for a much closer spatial arrangement of functional groups within the polymer structure compared to conventional methods that typically space them further apart. The newfound proximity creates innovative material characteristics that could lead to unique applications across multiple fields.
The researchers then employed polymerization techniques to link the modified monomers, resulting in polymers with custom-designed characteristics. The team acknowledges that traditional commercial polymers generally lack the advantages that come with the newly devised architecture, paving the way for a productive dialogue on how to enhance overall material performance. Dr. Ravn envisions experimenting with different functional groups in upcoming research to further understand their impact on material properties.
One of the most critical outcomes of this research is its potential contribution to sustainable practices in polymer production. Since nickel is relatively abundant compared to other metals commonly used in catalytic applications, the methodology presents a more environmentally friendly alternative to existing synthetic processes. The commitment to sustainability drives the researchers’ ambition to not only innovate new materials but also make the resultant products more eco-conscious.
Looking ahead, the team aims to explore strategies to degrade these newly synthesized long-chain polymers, reverting them back to their original monomeric forms. Such advancements would enable repurposing of the raw materials, aligning with global efforts to minimize waste and promote circular economies within the realm of chemical production.
The implications of this study extend far beyond mere theoretical advancements; they signify a paradigm shift in both polymer chemistry and material science. By introducing a versatile toolkit for creating new monomers, researchers are well-positioned to explore and craft polymers with novel functionalities that cater to modern technological needs. The innovation doesn’t merely enrich the academic landscape but also possesses the potential to revolutionize industries including healthcare, electronics, and energy storage.
The challenge of synthesized materials has taken a giant leap forward through collaborative efforts and novel approaches. As researchers continually leverage strategic partnerships and groundbreaking methodologies, the future of polymers appears not just bright but also ripe with sustainable promise, setting the stage for significant advancements in technology and environmental stewardship.

Leave a Reply