Organic molecules that exhibit chirality, or “handedness,” play a significant role in nature and have a wide range of applications in various fields. However, synthesizing molecules with the desired chirality in the laboratory has proven to be a challenging task. The inability to control the chirality of molecules accurately can render drugs or enzymes ineffective. Fortunately, a team of chemists from the University of California, Davis, led by Professor Dean Tantillo, has made significant progress in replicating nature’s efficiency in chiral molecule synthesis. Through a combination of computational modeling and physical experimentation, the researchers have successfully synthesized specific chiral molecules using rearrangements of simple hydrocarbons in the presence of complex organic catalysts.
In a groundbreaking study published in the journal Nature, Professor Tantillo and his colleagues from the Max Planck Institute in Germany detail their groundbreaking findings. Their research opens up new possibilities for harnessing hydrocarbons for a wide range of applications, including the development of pharmaceutical precursors and advanced materials. The team’s novel approach lies in their ability to selectively produce one chiral form of a molecule over its mirror image using a carbocation shift. This level of selectivity is unprecedented and presents exciting opportunities for synthetic chemists.
Challenges in Chiral Molecule Synthesis
Synthetic chemists often strive to create molecules with specific chirality, especially in drug development. This preference arises from the need for chiral molecules to selectively bind to proteins or enzyme targets. However, achieving this selectivity in a laboratory setting is fraught with challenges. Chiral molecules can be likened to “little balls of grease with some positive charge smeared around them,” making it difficult for chemical catalysts to bind to them in a specific orientation due to the lack of charged groups to grab onto.
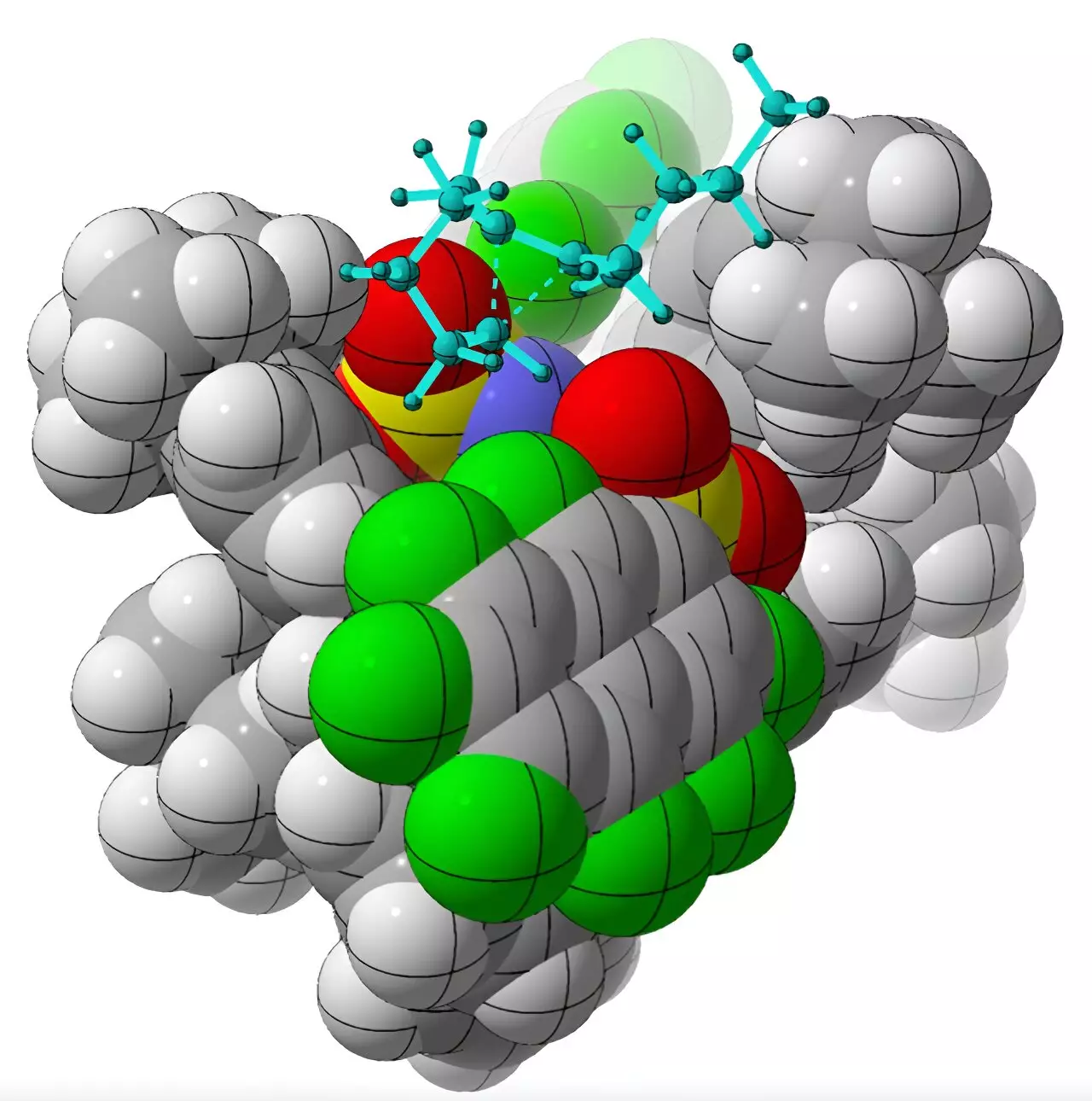
The chemists at the University of California, Davis, managed to overcome these challenges by using a chiral organic acid called imidodiphosphorimidate as a catalyst. This catalyst enabled the successful rearrangement of achiral alkenyl cycloalkanes, resulting in the production of chiral molecules called cycloalkenes. Through computational methods, Professor Tantillo and his team deciphered the underlying mechanism through which the catalyst selectively produces one chiral form over the other. The researchers drew parallels between the reaction and the behavior of enzymes involved in the natural synthesis of hydrocarbon products known as terpenes.
Mapping Terpene Reaction Pathways
Part of Professor Tantillo’s research involves mapping the reaction pathways of terpenes using quantum mechanical methods. By understanding the different possible pathways and the intermediates involved, researchers can gain insights into the formation of byproducts and impurities. This knowledge is crucial for the future engineering of terpene-forming enzymes and improving their efficiency. The successful application of computational methods in this study brings us one step closer to re-engineering terpene-forming enzymes and harnessing their potential in chiral molecule synthesis.
Expanding Molecular Possibilities
The new method developed by Professor Tantillo and his colleagues has the potential to synthesize both natural and non-natural chiral molecules. Currently, petroleum serves as a significant source of hydrocarbons. If researchers can catalytically convert these hydrocarbons into molecules with defined chirality, it would greatly increase the value and usefulness of these molecules. While the full extent of the practical applications of this breakthrough remains to be seen, the potential for advancements in pharmaceuticals, materials science, and other fields is undeniable.
The groundbreaking research conducted by Professor Dean Tantillo and his team at the University of California, Davis, represents a significant step forward in the synthesis of chiral molecules. By combining computational modeling with physical experimentation, the researchers have successfully achieved high selectivity in the production of specific chiral molecules. This breakthrough has the potential to revolutionize the field of organic chemistry, opening up new avenues for drug development, materials science, and beyond. The ability to harness hydrocarbons with precision and efficiency brings us one step closer to replicating nature’s unparalleled chemical complexity and unlocking its full potential.

Leave a Reply