Hydrogen gas has emerged as a frontrunner in the pursuit of renewable energy, primarily due to its high energy density and environmental benefits. As the most plentiful element in the universe, hydrogen presents unique opportunities for sustainable energy solutions. However, its natural form often binds with other elements, necessitating innovative extraction methods. One of the most prominent hydrogen carriers is ammonia (NH₃), which contains a significant amount of hydrogen—approximately 17.6% of its mass. This characteristic, combined with its abundance and ease of transportation, positions ammonia as a substantive part of the green energy landscape.
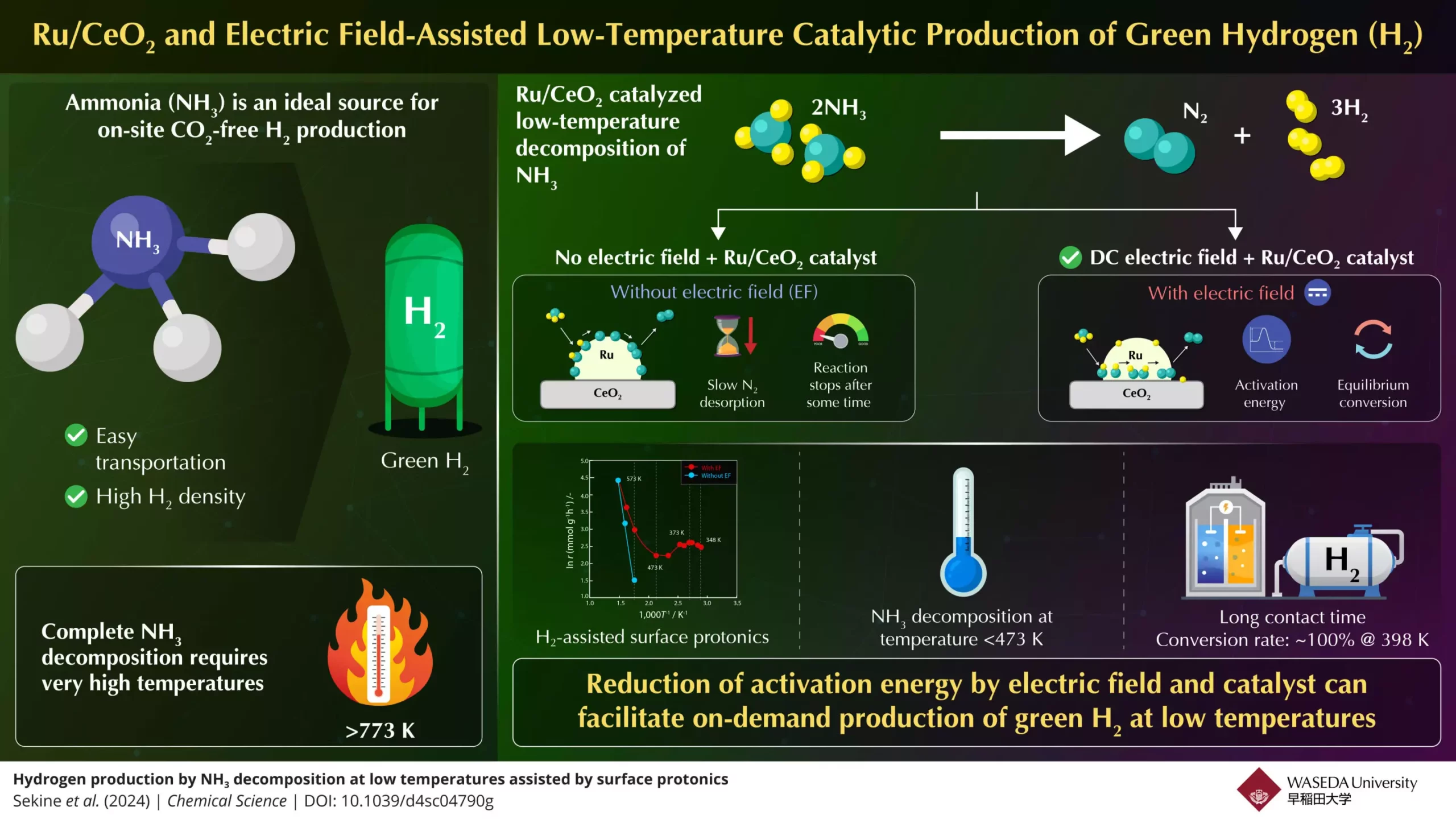
Despite this potential, the use of ammonia as a source of hydrogen faces considerable challenges. The decomposition of ammonia into hydrogen and nitrogen typically requires high temperatures—over 773K—making the process energy-intensive and thus less practical for on-demand applications, such as fuel cells or combustion engines. This indicates that there is a critical need for advancements in ammonia decomposition techniques to make hydrogen generation more efficient and viable for widespread use.
A significant breakthrough in this arena has been reported by a research team led by Professor Yasushi Sekine of Waseda University. Collaborating with experts from Yanmar Holdings, the team has initiated a shift in how ammonia can be decomposed into hydrogen at significantly lower temperatures. Their findings, published in Chemical Science, illustrate a novel experimental setup that leverages an electric field alongside a highly effective Ru/CeO₂ catalyst to facilitate ammonia conversion.
The core of their approach lies in the adjustment of reaction conditions to minimize the energy required for ammonia decomposition. Conventional methods have primarily focused on thermal catalysis, where high temperatures enable the necessary chemical reactions. However, through the application of an electric field, this team has shown that it is possible to enhance proton conductance at the catalyst’s surface. This innovation effectively lowers the activation energy needed for the reaction and permits efficient ammonia breakdown even below 473K, a temperature previously thought too low for effective conversion.
The team’s results are groundbreaking, demonstrating an impressive 100% conversion rate at 398K with enough contact time between the ammonia feed and the catalyst. This performance surpasses conventional equilibrium conversion rates under optimized conditions. Here, the role of the electric field is crucial; it promotes so-called surface protonics, enabling protons to hop along the catalyst surface, a mechanism that dramatically increases reaction efficiency.
Interestingly, the study has shown that when the electric field is not applied, the decomposition reaction stalls due to slow nitrogen desorption, underscoring the transformative influence of their methodology. Following this discovery, the research team has solidified the understanding that this electric field-assisted catalytic process is not just effective but essential for the advancement of ammonia decomposition techniques.
As the world searches for effective pathways to decarbonization, the implications of Sekine’s research are essential. By providing a means to generate clean hydrogen in a more efficient manner, this method could accelerate the transition to sustainable fuels. It stands to reason that enhanced ammonia decomposition at lower temperatures could facilitate the wider adoption of hydrogen as a fuel source in various applications, including transportation and industrial processes, without the overhead of extensive energy consumption typically associated with high-temperature techniques.
Innovative approaches that facilitate lower-temperature hydrogen production from ammonia not only have the potential to revolutionize the field but also symbolize a significant stride toward a greener future. As researchers continue to refine these techniques and enhance their implementation, the goal of establishing a sustainable, carbon-free energy economy becomes more attainable. Professor Sekine’s promising work signifies a step into a future where hydrogen energy can be harnessed conveniently and efficiently, further solidifying its place as a cornerstone in the shift toward a sustainable energy landscape.

Leave a Reply