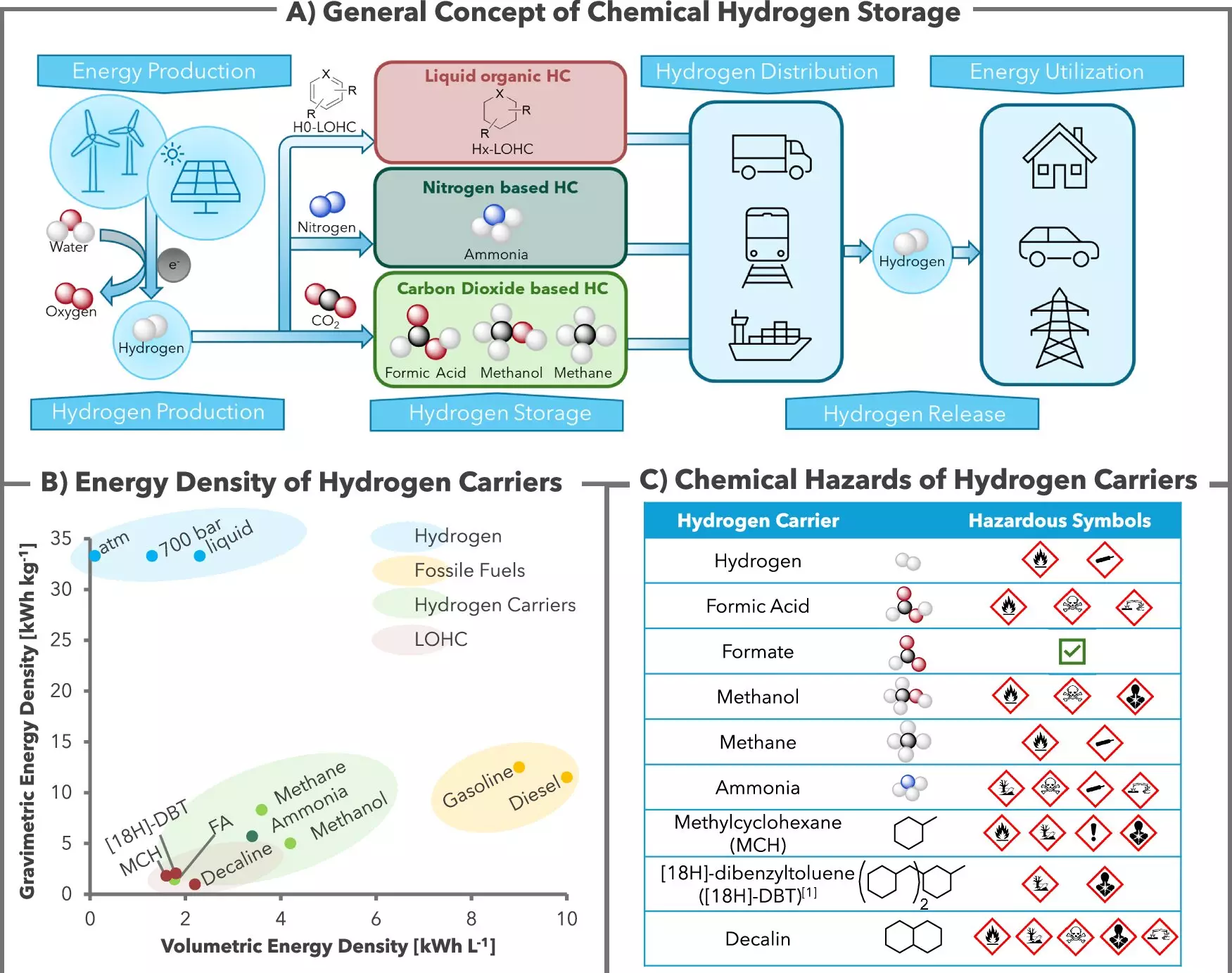
In the quest for sustainable energy sources, hydrogen has often been heralded as a pivotal player in the future energy landscape. It holds massive potential as a clean energy vector, but the challenges of safe and stable storage have kept it from realizing its full promise. Hydrogen’s volatile nature makes it difficult to handle, leading researchers to explore various methods to mitigate these dangers while unlocking its utility for energy storage and transport. In a significant advancement, a collaborative study between the Leibniz Institute for Catalysis (LIKAT) and H2APEX has unveiled an innovative approach to hydrogen storage that offers a promising solution.
Published in the prestigious journal Nature Communications, the research highlights a novel chemical storage method that leverages potassium bicarbonate—an everyday compound found in baking soda. The scientists have employed a homogeneous catalyst system, utilizing ruthenium as the active element, to enable hydrogen to bind with bicarbonate, thus forming a stable compound called formate. This method transforms hydrogen into a harmless salt, which can be easily stored and transported.
The significance of this research lies not only in its innovative use of commonly available materials but also in the efficiency and safety of the process. According to Dr. Rui Sang and Ph.D. student Carolin Stein, the pivotal aspect of this system is its reversibility; once the hydrogen is stored as formate, it can be readily extracted using the same catalyst, allowing for a simple and effective cycle of storage and release.
What sets this approach apart is its operational simplicity and safety profile. The reactions involved can occur at relatively low temperatures, around 60°C, ensuring energy efficiency throughout the process. The system’s design allows for technical control based on pressure, facilitating either the binding of hydrogen to bicarbonate or the release of hydrogen from formate—an essential feature for practical applications.
The safety aspect of using bicarbonate is noteworthy. Traditional methods for hydrogen storage or transport often necessitate more hazardous materials, raising concerns about toxicity. By using potassium bicarbonate and producing formate, researchers have managed to sidestep many of these issues, positioning this method as a safer alternative for future hydrogen economies.
One of the most critical applications of this hydrogen storage technique is its potential integration into renewable energy systems. As the world leans toward wind and solar power, the ability to produce green hydrogen through electrolysis becomes increasingly viable. During periods of surplus energy—when the production from renewable sources exceeds consumption—hydrogen can be generated and subsequently stored as formate, creating a flexible energy management strategy akin to charging a battery.
This method is especially advantageous for rural areas, where electricity production might fluctuate due to variable weather conditions. By using hydrogen stored in formate, localized energy systems can become self-sufficient and resilient, directly addressing the intermittency challenges that plague renewable energy sources.
With over 40 successful storage and release cycles documented, the research team has proven the feasibility of this approach. They achieved impressive results, generating 50 liters of hydrogen with an average purity exceeding 99.5% while consuming minimal ruthenium catalyst. These accomplishments are paving the way for H2APEX to build a larger-scale demonstrator, with the aim of commercializing this technology by the end of 2025.
The transition to a hydrogen economy rests on innovation like this, where the basic chemical principles are utilized to create efficient, safe, and sustainable energy solutions. The researchers emphasize that their method is CO2-neutral, eliminating concerns around carbon emissions typically associated with hydrogen extraction processes.
The groundbreaking work by LIKAT and H2APEX marks a significant step in the ongoing journey toward effective hydrogen storage solutions. By using potassium bicarbonate to safely and efficiently bind hydrogen, the researchers have opened new avenues for the utilization of this abundant energy carrier. As the world grapples with the need for a clean energy transition, innovations like this provide a glimpse of hope for a sustainable future, where hydrogen becomes a vital component of our energy systems. In essence, the developments in this area do not just yield technical advancements; they also embody an aspirational shift in how we can harness the energy of the future.

Leave a Reply