In a groundbreaking study conducted at the University of Illinois Urbana-Champaign, researchers have made significant strides in understanding the role of solvation in ion binding. Their findings present a new pathway for electrochemically controlling ion selectivity, opening up possibilities for innovative applications in various fields. Led by Chemical & Biomolecular Engineering professor Xiao Su and Ph.D. student Raylin Chen, the team’s research builds upon their previous work in electrochemical separations and sheds light on the critical mechanism of solvation in binding ions.
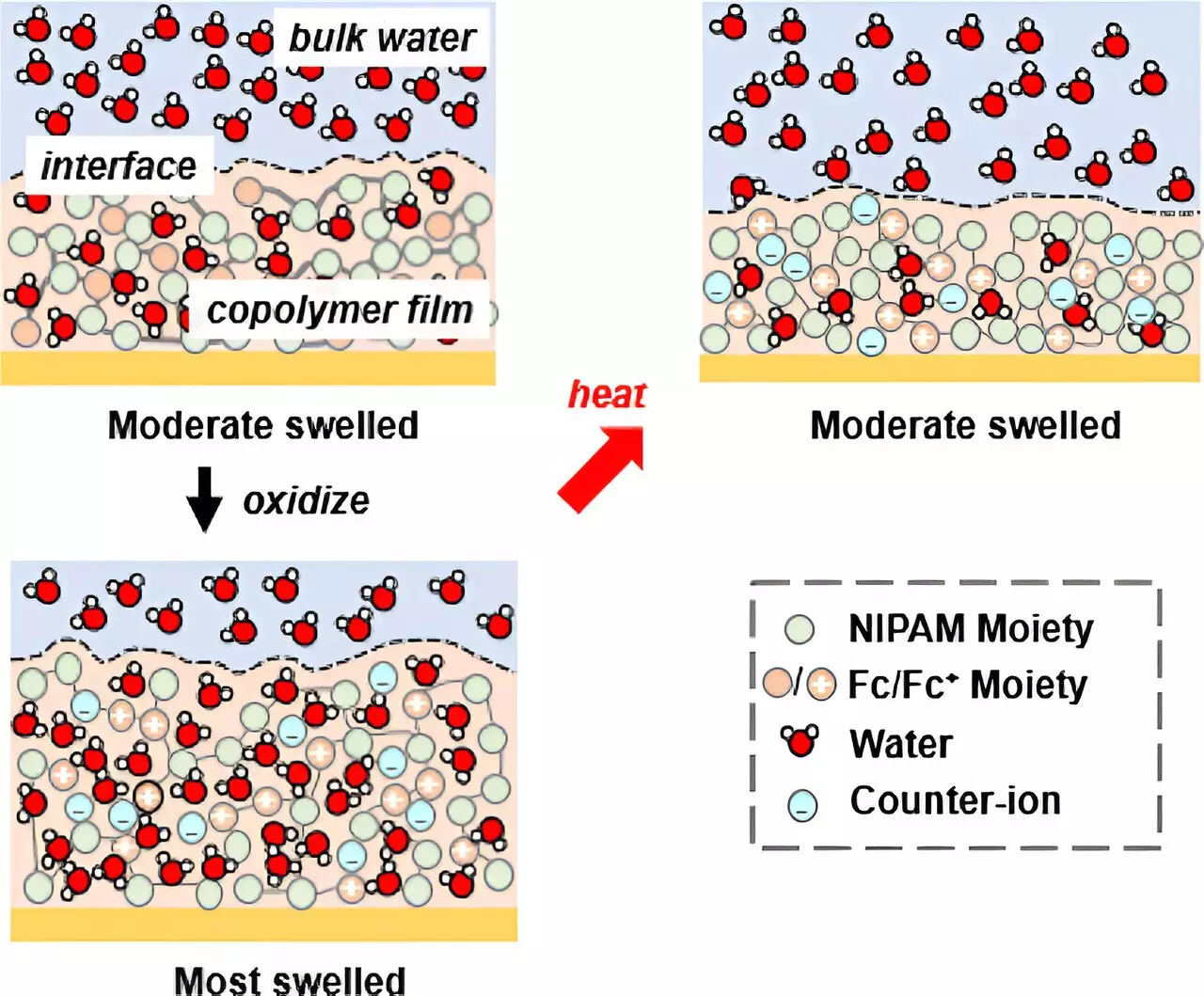
Solvation, the process of solute molecules being surrounded and stabilized by solvent molecules, has long been recognized as a crucial factor in ion binding. To gain a deeper understanding of this phenomenon, the researchers sought to control the solvation of a polymer and leverage it for specific ion binding. Their novel approach involved a copolymer system incorporating N-isopropyl acrylamide (NIPAM), known for its temperature-responsive properties, along with redox-active units.
By introducing redox-active units to the copolymer system, the researchers created a unique platform with two distinct pathways for controlling solvation. They discovered that by modulating the electrochemical potential, they could effectively manipulate the solvation behavior of the NIPAM unit. Instead of relying on a thermal transition, they triggered an electrochemical transition in the NIPAM unit. This breakthrough allowed them to selectively bind different ions based on the degree of solvation, revolutionizing the current understanding of ion selectivity.
With the copolymer system yielding gel films, the researchers developed a platform for solvation-controlled ion separations. They employed an innovative method called in situ ellipsometry, enabling them to observe the film’s thickness as it swelled or deswelled in response to the addition or release of water through electrochemistry.
Collaborating with a team from Oak Ridge National Laboratory, led by Jim Browning, Hanyu Wang, and Mat Doucet, the researchers utilized neutron reflectometry (NR), a technique capable of visualizing hidden features within materials. The unique sensitivity of neutrons to water distribution facilitated the observation of solvation across the film. Under specific electrochemical potentials, the film swelled and absorbed water, providing valuable insights into controlling ion selectivity through precise solvation control.
Implications for Sustainable Technologies
One of the most promising aspects of this research is its potential applicability in various sustainable scenarios. The ability to activate the copolymer system by both temperature and electrochemical potential sets the stage for the development of materials platforms that can be powered by renewable energy sources or waste heat.
The researchers’ findings have significant implications for water treatment and resource recovery, showcasing the potential for more energy-efficient and selective technologies. By gaining a deeper understanding of the molecular mechanisms involved in ion binding, this study opens up avenues for the development of more precise and efficient technologies.
The study conducted by researchers at the University of Illinois Urbana-Champaign marks a significant advancement in the field of solvation-controlled ion selectivity. By uncovering the critical role of solvation in ion binding and introducing a novel electrochemical approach, the researchers have paved the way for future developments in various sustainable applications. Their work not only enhances our understanding of the molecular mechanisms at play but also offers a platform for the creation of more energy-efficient and selective technologies. As we continue to strive for advancements in water treatment and resource recovery, the control of ion binding mechanisms becomes increasingly vital, driving us towards a future of heightened precision and sustainability.

Leave a Reply