The utilization of CO2 as a chemical raw material holds immense potential for reducing emissions and decreasing the dependency on fossil feedstocks. A recent study published in the journal Angewandte Chemie International Edition introduces a groundbreaking metal-free organic framework that enables the electrocatalytic production of ethylene from CO2. Ethylene, a crucial building block for various products such as polyethylene and plastics, is traditionally obtained through energy-intensive processes involving fossil feedstocks. However, the electrochemical conversion of CO2 to ethylene offers a promising pathway to not only curb CO2 emissions but also conserve energy and fossil resources.
CO2 is a highly stable molecule, posing a significant challenge in inducing it to undergo chemical reactions. Through the application of electricity and catalysts, researchers have succeeded in converting CO2 into C1 chemicals like methanol and methane. However, the production of ethylene presents an additional hurdle, as it requires the formation of a bond between two carbon atoms, a feat previously accomplished only with copper-based catalysts. The development of a metal-free electrocatalyst for ethylene production signifies a significant advancement in the field, offering economic and environmental benefits by eliminating the reliance on costly metals and potentially hazardous environmental consequences.
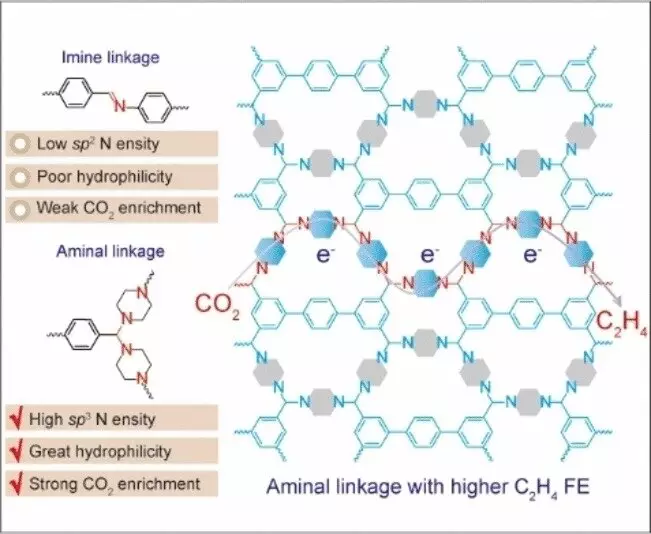
A team of researchers led by Chengtao Gong and Fu-Sheng Ke at Wuhan University in China has engineered a metal-free electro-catalyst for converting CO2 to ethylene, based on a nitrogen-containing covalent organic framework (COF). In contrast to metal-organic frameworks (MOFs), COFs are purely organic materials characterized by their porous and crystalline nature, offering tailored chemical properties without the need for metal ions to stabilize the structure. The newly developed COF incorporates nitrogen atoms with a distinct electron configuration (sp3 hybridization) as catalytically active sites, facilitating the binding of building blocks into a framework through aminal links.
Aminal-linked COFs exhibit unique structural features, demanding precise bond lengths and angles between building blocks, leading to the formation of frameworks through ring closures. Utilizing piperazine and a building block composed of aromatic carbon rings, the researchers achieved a successful combination that resulted in high selectivity and performance for ethylene production. The presence of active sp3 nitrogen centers within the COF enables efficient CO2 capture and electron transfer, leading to the formation of excited intermediates capable of undergoing C-C coupling. In contrast, COFs containing sp2 nitrogen failed to produce ethylene, underscoring the significance of proper electron configuration in the electrochemical reduction of CO2 to ethylene.
The development of metal-free electro-catalysts based on nitrogen-containing COFs represents a significant step towards sustainable chemical production, offering a more environmentally friendly and cost-effective alternative to traditional methods reliant on metal catalysts. By harnessing the unique properties of COFs and optimizing the electron configuration of nitrogen centers, researchers have unlocked a pathway for transforming CO2 into valuable chemical products like ethylene. Moving forward, continued research in this area holds the potential to revolutionize the chemical industry, paving the way for greener and more sustainable manufacturing processes.

Leave a Reply