Ammonia, a crucial compound used in fertilizers, has long been produced through the energy-intensive Haber-Bosch process. However, researchers at the RIKEN Center for Sustainable Resource Science (CSRS) in Japan, led by Satoshi Kamiguchi, have recently discovered a more sustainable way to synthesize ammonia. This groundbreaking study, published in Chemical Science, unveils a new catalyst that operates at lower temperatures, reducing the energy and financial resources required for ammonia production.
Traditional ammonia production involves high pressure and high temperatures, consuming a significant amount of energy. The new catalyst developed by the RIKEN CSRS researchers offers a more eco-friendly alternative by allowing for stable reactions at lower temperatures. This innovation not only lowers the energy footprint of ammonia production but also paves the way for a transition to a carbon-neutral and green-energy economy.
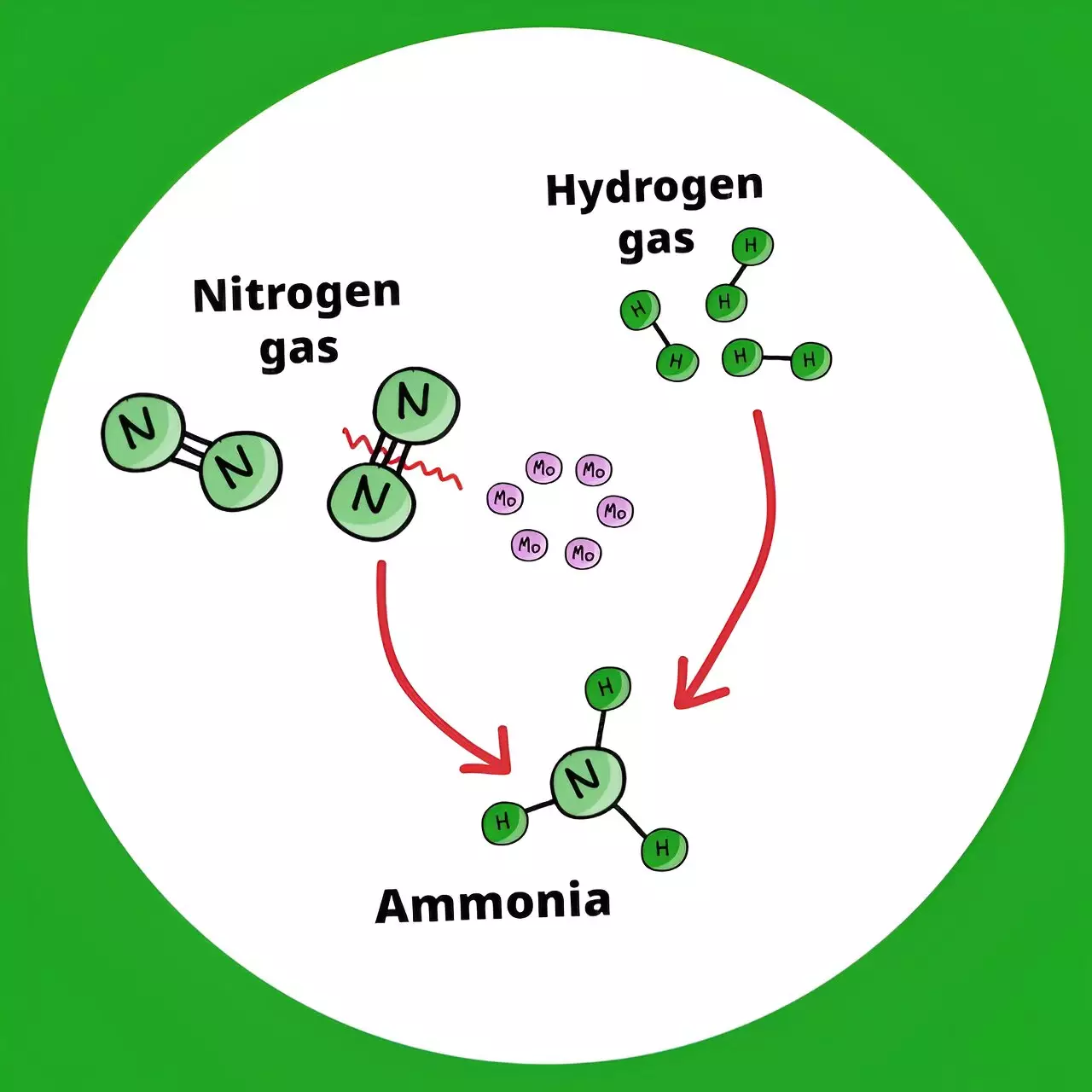
The Science Behind the Discovery
Breaking down nitrogen gas, which has a strong triple bond between its atoms, was a major challenge in developing the new catalyst. By utilizing ultrasmall molybdenum metal particles activated with hydrogen gas, the researchers were able to overcome this hurdle. The activated molybdenum atoms efficiently break the nitrogen-nitrogen bonds, accelerating ammonia synthesis. The results of the study showed continuous production of ammonia at significantly lower temperatures than the conventional Haber-Bosch process.
The impact of this new method extends beyond the fertilizer industry. Ammonia fuel, which can be burned in internal combustion engines without emitting CO2, could become a practical alternative with the introduction of the energy-efficient catalyst. By reducing the energy requirements of ammonia production, the new process has the potential to significantly decrease carbon emissions on a global scale.
Challenges and Opportunities
While the new catalyst presents a promising solution for greener ammonia production, challenges still exist. The reliance on fossil fuels for hydrogen production remains a significant source of CO2 emissions and energy consumption. Kamiguchi emphasizes the importance of integrating the catalyst system with green hydrogen production from renewable sources to further reduce carbon emissions and combat global warming.
As the research team continues to explore ways to enhance the efficiency of the molybdenum-based catalyst, the future of sustainable ammonia production appears promising. By leveraging this innovative approach, the global energy landscape could undergo a transformation towards decreased carbon emissions and increased sustainability. The potential of green ammonia as a carrier for hydrogen fuel opens up new possibilities for a cleaner and more efficient energy future.

Leave a Reply