Calcite, a crystalline form of calcium carbonate, is a mineral with a rhombohedral appearance that is abundant on Earth. As a major component of limestone and marble, calcite is known for its stability compared to other forms of calcium carbonate, such as aragonite and vaterite. The internal structure of calcite is a key factor in understanding its properties and reactivity.
Recent research has shed light on how different synthesis approaches can dramatically alter the internal structure of calcite particles. This discovery has significant implications for various applications, including environmental remediation, material development, and catalysis. Understanding the relationship between synthesis methods and internal defects in calcite is crucial for harnessing its full potential.
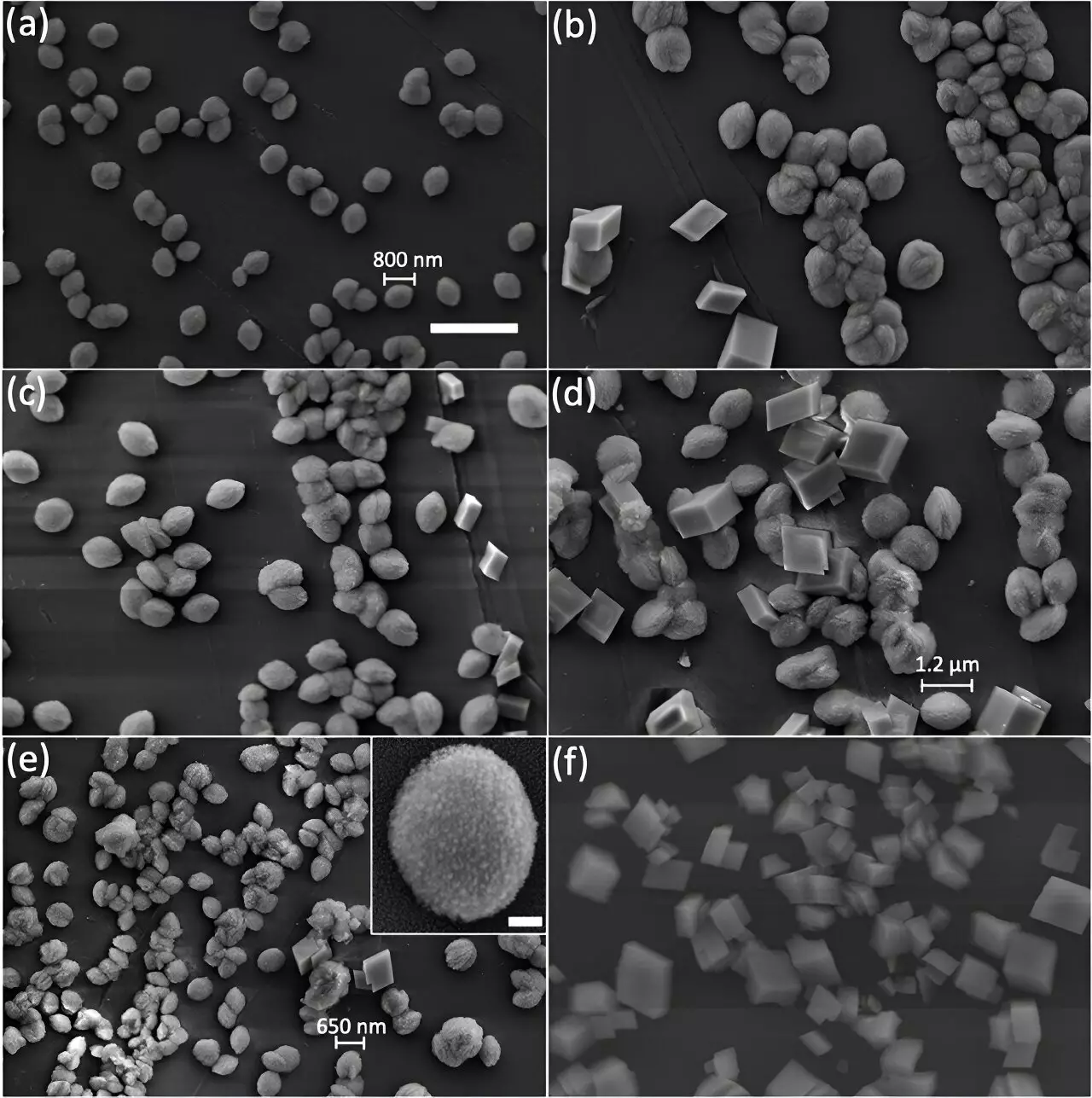
A study conducted by researchers at Argonne National Laboratory focused on visualizing the internal structure of calcite particles grown using two distinct synthesis approaches. By utilizing advanced imaging techniques like scanning electron microscopy (SEM) and Bragg Coherent Diffraction Imaging (BCDI) at the Advanced Photon Source (APS), the researchers were able to observe nanoscale defects within the calcite crystals.
The study compared calcite crystals grown slowly to those grown quickly and found significant differences in their internal structure. While slow-grown crystals exhibited uniform patterns and shapes, quick-grown crystals revealed nanosized defects and fragmented structures. This discovery challenges the traditional perception of calcite as a perfectly ordered mineral and highlights the role of synthesis speed in creating internal defects.
The presence of nanoscale defects in calcite has profound implications for its reactivity and functionality. Internal fragmentation can influence how calcite interacts with its environment, including its ability to absorb toxic substances like heavy metals and its mechanical strength. By understanding and utilizing these defects, researchers can tailor calcite for specific applications, such as improving catalysts and designing durable materials.
Moving forward, the research on calcite synthesis and internal structure opens up new possibilities for controlling mineral reactivity and designing novel materials. By leveraging nanoscale defects and understanding their impact on calcite properties, scientists can optimize the performance of calcite-based products in various fields. The ability to distinguish between perfect and flawed calcite crystals provides a valuable tool for engineering materials with enhanced properties.
The study of calcite synthesis and internal structure represents a significant advancement in mineral science. By exploring the relationship between synthesis approaches and nanocrystalline defects, researchers have uncovered new insights into the reactivity and functionality of calcite. This knowledge has the potential to drive innovation in fields such as environmental science, materials engineering, and catalysis.

Leave a Reply