The attachment of carbohydrates, or glycans, onto proteins and lipids is a fundamental process within the human body that plays a crucial role in various physiological functions. When this process malfunctions, the risk of developing diseases such as cancer, diabetes, Alzheimer’s, and muscular dystrophy significantly increases. Glycans are essential for cell recognition, cell signaling, immune response, protein folding, development, and fertilization. Due to the importance of glycans, researchers have established a field known as glycobiology to study the structure and function of these molecules and their associated processes.
Despite the advancements in glycobiology, researchers and clinicians still struggle to accurately predict and fine-tune glycan structures within cells. This limitation hinders their ability to effectively respond to diseases caused by faulty glycosylation processes. To address this issue, it is crucial to understand how enzymes called glycotransferases, responsible for attaching glycans to proteins or lipids, are regulated within cells. By gaining insight into the enzymatic activity involved in glycan attachment, researchers can better identify disease mechanisms and potential therapeutic targets.
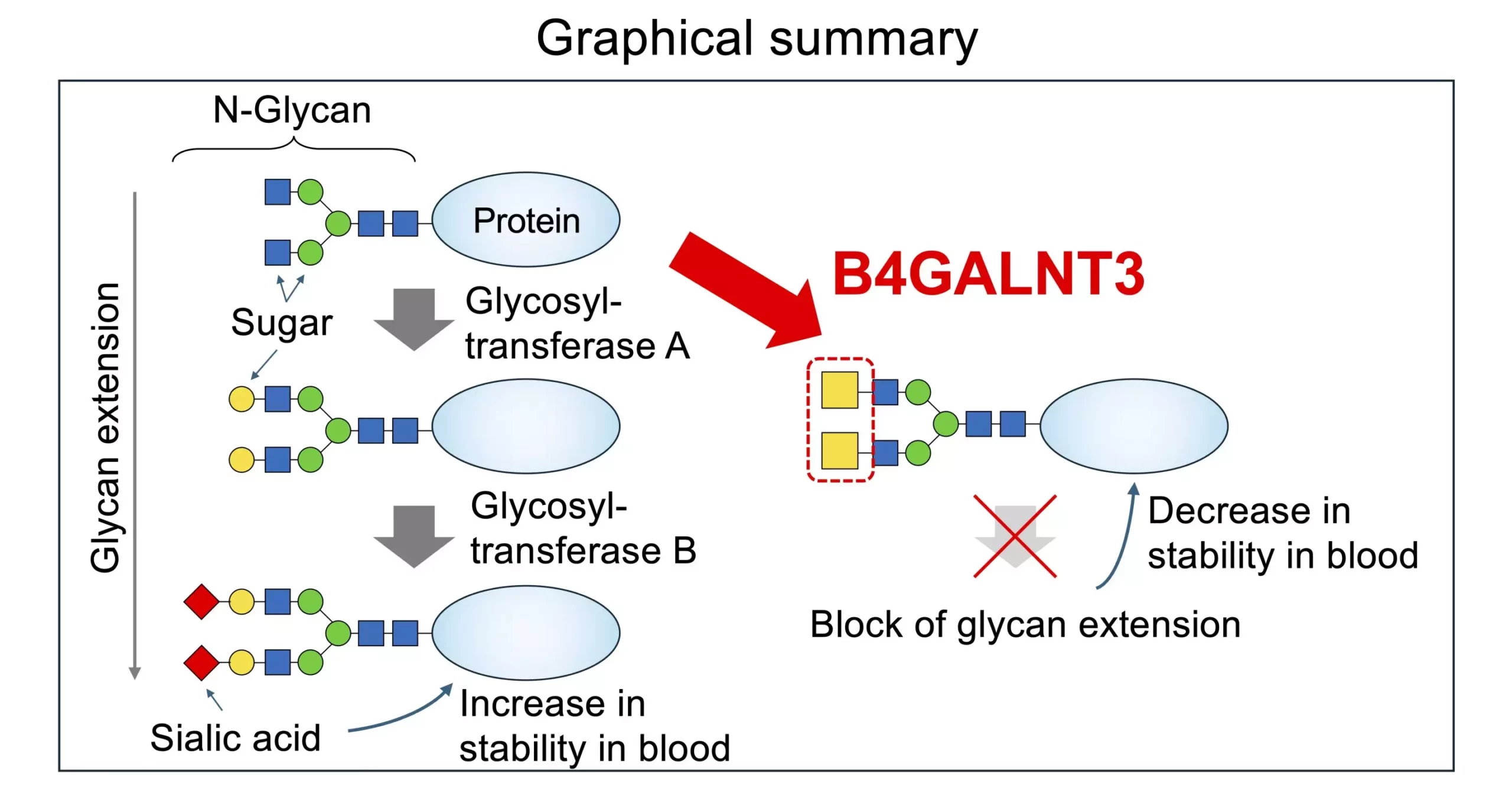
Within the structure of glycans, a two-sugar entity known as LacdiNAc (LDN) plays a significant role in various bodily processes and disease development. The enzymes B4GALNT3 and B4GALNT4 catalyze the biosynthesis of the LDN structure, which has been linked to hormone modification, maintenance of stem cells, and alteration of bone density. Dysregulation of LDN production has been associated with a higher risk of fractures, colon cancer, and other diseases. Understanding the synthesis and function of LDN is crucial for unraveling disease pathogenesis and identifying targets for therapeutic intervention.
Recent advancements in structural biology, enzymology, and glycomics have provided researchers with tools to investigate the role of enzymes in regulating glycan structures. By analyzing the structures of glycosyltransferases, such as B4GALNT3, researchers have identified essential domains, such as the PA14 domain, that influence enzyme activity. Mutational analyses of glycosyltransferases lacking the PA14 domain revealed a significant decrease in enzymatic activity related to glycan production, highlighting the essential role of this domain in the process.
The presence of LDN in glycan structures has been shown to have a distinct impact on the modification of sugar residues at the terminals of N-glycans compared to the LacNAc structure. LDN affects the production of N-glycans by influencing the activities of glycosyltransferases involved in glycoprotein modification. This finding underscores the importance of understanding the structural variations of glycans and their implications for disease development.
The regulation of glycan structures through enzymatic processes plays a critical role in disease development and pathogenesis. By uncovering the intricacies of glycan biosynthesis and enzyme activity, researchers can identify potential therapeutic targets for diseases associated with faulty glycosylation. Further exploration of the role of enzymes like B4GALNT3 and the impact of structures like LDN on disease progression is essential for advancing our understanding of glycobiology and developing targeted interventions for a range of disorders.

Leave a Reply