Glycosylation is a crucial process that involves attaching carbohydrates to the backbone of a protein through enzymatic reactions. This process has a significant impact on protein structure, function, and stability, making it a critical area of study in the field of biochemistry. Researchers at Boston University Chobanian & Avedisian School of Medicine recently conducted a study to enhance the identification of glycopeptides and their associated glycans. The findings of this study shed light on the challenges faced in identifying peptides with multiple glycans and emphasize the potential therapeutic opportunities that could arise from a deeper understanding of glycosylation and its implications in various diseases.
The Types of Protein Glycosylation
There are two primary types of protein glycosylation: N-glycosylation and O-glycosylation. N-glycosylation involves the attachment of carbohydrates to the nitrogen atom of asparagine residues, while O-glycosylation refers to the attachment of carbohydrates to the oxygen atom of serine or threonine residues. Both forms of glycosylation play essential roles in protein function and have distinct characteristics that influence their identification and analysis.
The researchers discovered that conventional mass spectrometry methods were effective in identifying peptides with a single glycan. However, when it came to peptides with two or more glycans, an additional step was necessary. This poses a challenge as proteins can carry multiple glycans, and it is difficult to generate peptides that only contain one glycan. The ability to identify peptides with multiple glycans is crucial in unraveling different glycoforms of proteins and understanding their biological functions and disease implications.
The lack of comprehensive information about glycoproteins and their alterations during disease development has hindered the identification of potential therapeutic avenues. Manveen K. Sethi, Ph.D., a research assistant professor of biochemistry & cell biology and the corresponding author of the study, believes that a better understanding of how glycosylation impacts diseases could lead to novel treatment targets. By identifying site-specific alterations in glycosylation, researchers can differentiate healthy and diseased states and identify glycoproteins that could serve as markers or therapeutic targets in various diseases.
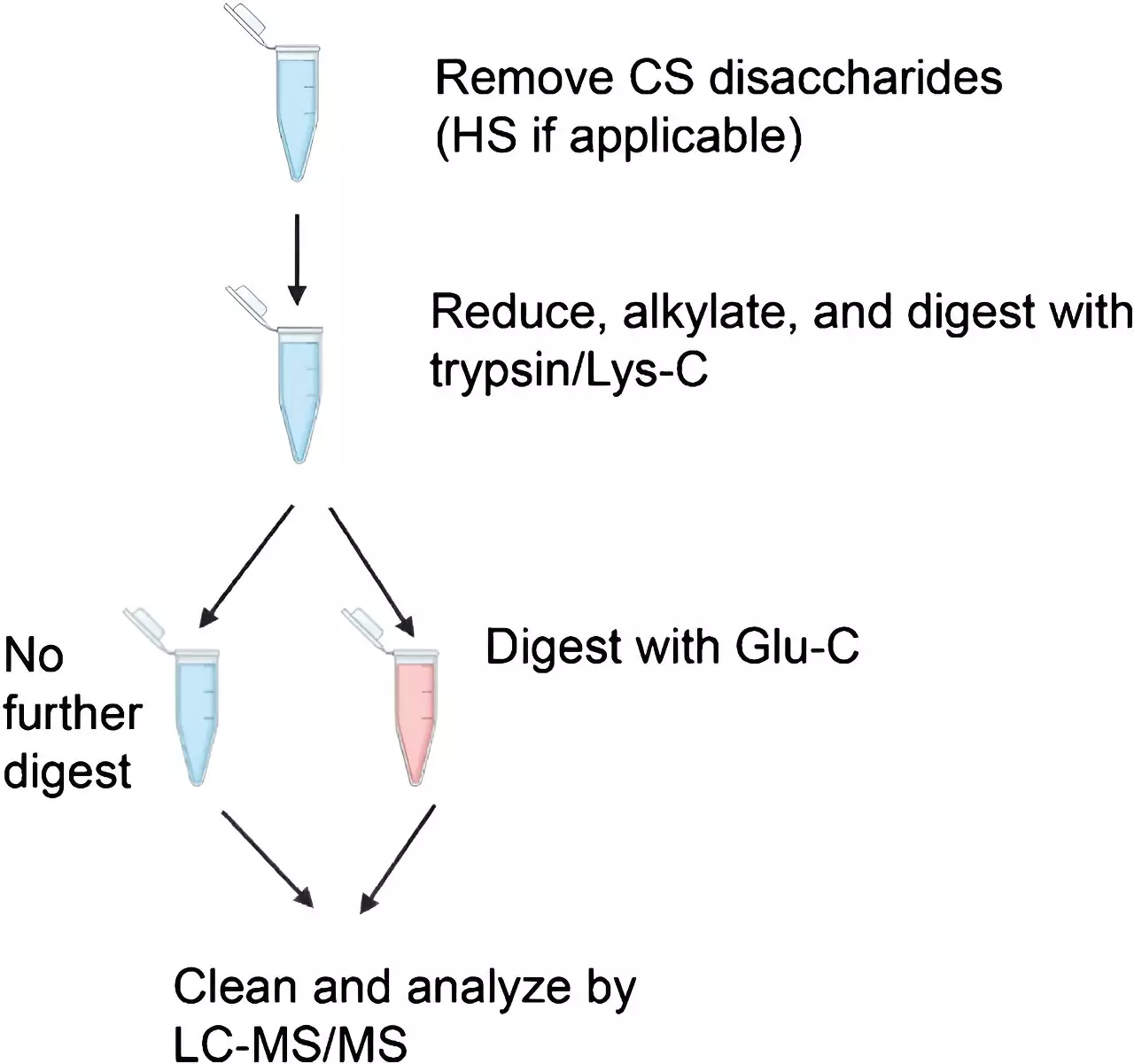
To improve the identification of glycopeptides, the researchers utilized different enzymatic digestion protocols and mass spectrometry methods. Using four standard proteins, they compared the effectiveness of conventional higher-energy collisional dissociation (HCD) and a more recently developed method known as electron transfer/higher energy collisional dissociation (EThcD). While HCD was sufficient in identifying glycopeptides, EThcD proved essential in detecting glycans, especially in the case of multiply-glycosylated peptides.
The study conducted by researchers at Boston University Chobanian & Avedisian School of Medicine highlights the significance of glycosylation in protein function and disease pathogenesis. By improving methods to identify glycopeptides and multiple glycans, researchers can gain a deeper understanding of protein glycoforms and their role in various diseases. This knowledge opens up new avenues for therapeutic interventions and the development of targeted treatments. Further advancements in technology and analytical techniques will undoubtedly contribute to enhancing our understanding of glycosylation and its implications in human health and disease.

Leave a Reply