As humans, we are often intrigued by the complex behavior associated with living organisms. The interplay between different entities can be mesmerizing, prompting us to question the fundamental forces that govern these interactions. Recent research by the University of Maine and Penn State has shed light on non-reciprocal interactions at the molecular level, challenging our conventional understanding of how molecules interact. In this article, we delve into the fascinating world of non-reciprocal interactions and the mechanisms behind them.
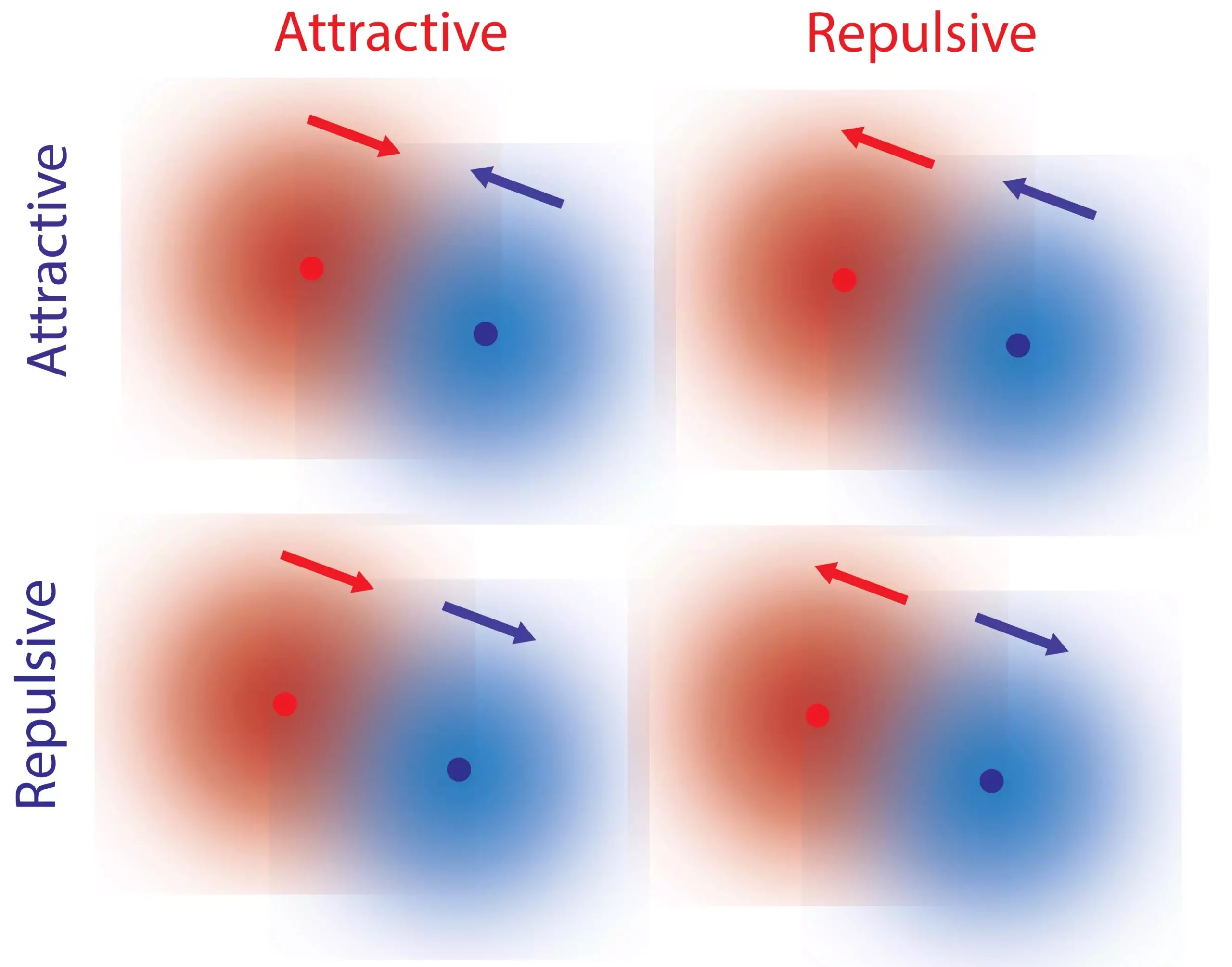
Traditionally, fundamental forces such as gravity and electromagnetism have been described as reciprocal. Objects are either attracted to each other or repelled by one another, adhering to the principle of reciprocity. However, our everyday experiences highlight interactions that do not conform to this law. Consider the relationship between a predator and its prey – while the predator is attracted to the prey, the prey tends to flee from the predator. These non-reciprocal interactions are essential for the intricate behavior observed in living organisms.
Previously, scientists explained non-reciprocal interactions in microscopic systems, such as bacteria, through hydrodynamic or external forces. These forces were believed to also underpin interactions between single molecules. However, the research published in Chem by UMaine’s R. Dean Astumian and collaborators Ayusman Sen and Niladri Sekhar Mandal introduces a novel mechanism that challenges this previous understanding.
The researchers identified local gradients of reactants and products as crucial elements in facilitating non-reciprocal interactions between single molecules. Every chemical catalyst, including biological catalysts like enzymes, induces reactions and creates these gradients. Herein lies a fascinating property known as kinetic asymmetry.
Through careful analysis and discussions, the researchers made a breakthrough “Eureka moment” when they realized that kinetic asymmetry, a property inherent to catalysts, controls the direction of response to a concentration gradient. This means that a particular molecule can repel one molecule while attracting another, leading to non-reciprocal interactions. Importantly, kinetic asymmetry is not fixed and can undergo evolution and adaptation, highlighting its significance in complex biological processes.
Previous research in the field of “active matter” has explored the consequences of non-reciprocal interactions. However, these studies typically introduced ad hoc forces to induce such interactions. The work by Mandal, Sen, and Astumian goes a step further by uncovering the molecular mechanisms that naturally give rise to non-reciprocal interactions between single molecules.
The implications of kinetic asymmetry extend beyond understanding the intricate dance of molecules. This property also plays a vital role in directing biomolecular machines and has even influenced the design of synthetic molecular motors and pumps. By investigating loose associations of different catalysts, Astumian, Sen, and Mandal aim to unravel the organizational principles that may have kick-started the formation of the earliest metabolic structures, ultimately leading to the evolution of life.
We find ourselves at the beginning stages of this exciting research, as scientists strive to understand the underpinnings of non-reciprocal interactions at the molecular level. The discovery of kinetic asymmetry opens up new avenues for exploring the origins of life and sheds light on the complex processes that transform simple matter into the intricate web of life we see today. As we delve deeper into the fundamental forces shaping our world, we might uncover even more intriguing mysteries waiting to be unraveled.

Leave a Reply