Iridium oxide catalysts have shown great promise in the realm of green technologies, particularly in the field of water oxidation. This makes them highly sought after for applications aimed at reducing our reliance on fossil fuels. A recent study published in the Journal of the American Chemical Society delved deep into the inner workings of these catalysts, shedding light on how they facilitate the crucial oxygen evolution reaction (OER). This research was spearheaded by a team of experts from SANKEN at Osaka University, who utilized advanced spectroscopy techniques to gain unprecedented insights into the behavior of chemical species involved in the OER.
The OER holds immense importance in various clean energy processes, such as the conversion of carbon dioxide into liquid fuels and the generation of green hydrogen through water electrolysis. These processes are essential for paving the way towards a sustainable future devoid of fossil fuels. As such, understanding the intricacies of the OER has become a focal point of research in the scientific community.
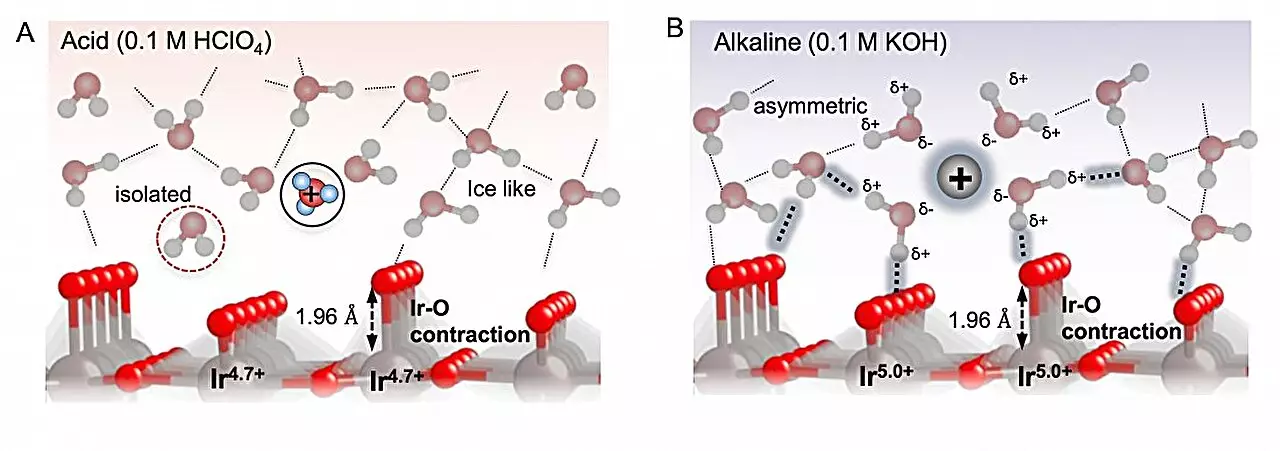
Catalytic processes are inherently complex, involving multiple intermediate species that play a role in transforming raw materials into desired end products. Operando techniques offer a unique opportunity to observe these intermediates in action using spectroscopy while the reaction is ongoing. By employing an electrode coated with iridium oxide, the researchers were able to study how water molecules undergo oxidation in solutions with varying pH levels.
One of the key factors in catalytic efficiency is the binding of reaction intermediates to the electrode surface. This binding must strike a delicate balance – tight enough to facilitate interactions with the electrode, yet not so strong that the intermediates become immobilized. The team discovered that long-range interactions between intermediates through the solution play a crucial role in controlling this binding, a phenomenon influenced by the pH of the environment.
Under alkaline conditions, the proximity of water molecules to the electrode surface was found to influence the long-range interactions between oxygenated species. This had a direct impact on their binding to the surface – while intermediates exhibited stronger binding at higher pH levels, the presence of interfacial water destabilized the oxygenated species, facilitating the reaction process.
By employing operando spectroscopy in conjunction with complementary techniques, the researchers were able to gain a comprehensive understanding of catalyst performance beyond mere electrode binding. This valuable insight is expected to play a pivotal role in optimizing the kinetics of the OER, thereby enhancing the efficiency of water oxidation for green hydrogen production.
The findings from this study have far-reaching implications, not only in the realm of water oxidation but also in catalyzing various other processes. The combination of operando spectroscopy with complementary techniques holds immense potential for unraveling the mysteries of catalysis and paving the way for a more sustainable future.

Leave a Reply