Fungal infections pose a growing public health crisis, and finding effective treatments has become increasingly urgent. In a groundbreaking study published in the journal Nature, researchers from the University of Illinois Urbana-Champaign and the University of Wisconsin-Madison have developed a new antifungal molecule that shows promise in combating fungal infections. By modifying the structure of the well-known antifungal drug Amphotericin B, the researchers have successfully eliminated its toxicity while harnessing its potent antifungal properties.
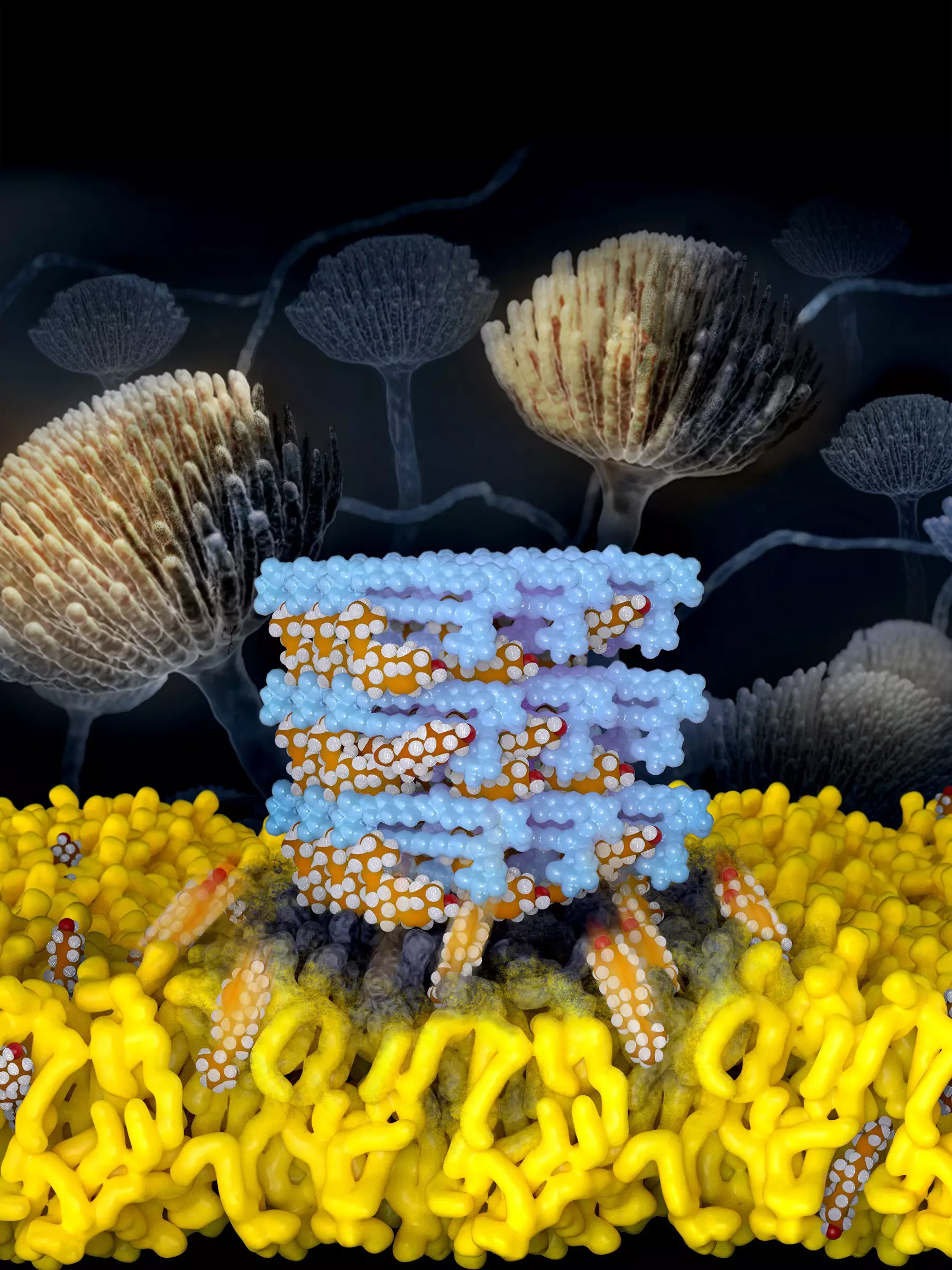
Amphotericin B, a naturally occurring small molecule produced by bacteria, is known for its ability to kill fungi. However, its use is limited due to its toxicity to human patients, particularly the kidneys. Recognizing the need for a safer and more effective treatment, Dr. Martin D. Burke, an Illinois professor of chemistry and a professor in the Carle Illinois College of Medicine, spearheaded this research. His team aimed to develop a derivative of Amphotericin B that could selectively target and kill fungi without causing harm to humans.
In previous studies, Burke’s group utilized a building block-based approach to molecular synthesis and collaborated with a team specializing in molecular imaging tools at the University of Wisconsin-Madison. Together, they uncovered the mechanism of Amphotericin B: the drug acts as a sponge to extract ergosterol, a vital component of fungal cells. However, they also discovered that Amphotericin B extracts cholesterol, the most common sterol in humans, from kidney cells, leading to its toxicity.
Through atomic-level structure analysis, the researchers identified subtle differences in the binding interactions between Amphotericin B and each sterol. This crucial insight enabled them to modify Amphotericin B and tune its binding properties, reducing its interaction with cholesterol and minimizing its toxicity. Armed with this knowledge, the Illinois team embarked on synthesizing and testing derivatives of Amphotericin B.
The researchers extensively tested the synthesized derivatives, evaluating their efficacy in killing fungi and assessing their toxicity on in vitro assays, cell cultures, and live mice. Among the molecules investigated, one stood out from the rest: AM-2-19. This derivative demonstrated kidney-sparing properties, strong resistance evasion, and broad-spectrum efficacy against over 500 clinically relevant pathogen species. It either matched or surpassed the efficacy of currently available antifungal drugs.
To assess its safety, the researchers tested AM-2-19 on human blood and kidney cells. They also conducted tests on mouse models of three common and stubborn fungal infections, where AM-2-19 displayed high efficacy. Renowned for its harsh impact on patients, Amphotericin B was often referred to as “ampho-terrible.” However, the development of AM-2-19 has the potential to transform it into “ampho-terrific” by decoupling efficacy from toxicity.
While the researchers are enthusiastic about the potential of AM-2-19, they emphasize the need for clinical studies to validate its effectiveness in humans. A critical step in this direction has been the licensing of AM-2-19 to Sfunga Therapeutics, which has recently initiated Phase 1 clinical trials.
The development of AM-2-19 marks a significant milestone in the quest for safer and more effective antifungal treatments. By leveraging the power of nature and applying cutting-edge scientific techniques, researchers have successfully overcome the toxicity limitations of Amphotericin B. The potential of AM-2-19 to revolutionize the treatment landscape for fungal infections is promising, but further studies are necessary to determine its efficacy and safety in human patients.
As fungal infections continue to pose a growing threat to public health, the development of novel antifungal agents is of paramount importance. The success of AM-2-19 highlights the transformative potential of deepening our understanding of fundamental science and harnessing nature’s tools. With ongoing research and clinical trials, the day may come when fungal infections no longer hold the same threat they do today, thanks to the groundbreaking work of dedicated scientists and the revolutionary potential of AM-2-19.

Leave a Reply