When it comes to understanding the intricate workings of enzymes, particularly copper amine oxidases, a class of enzymes critical in the healing and detoxification processes within our bodies, researchers face numerous challenges. One such challenge is identifying the precise positions of hydrogen atoms within these enzymes, which is crucial for developing improved versions with enhanced biochemical reactivity. However, commonly used technologies have limitations in providing this level of detail.
In a groundbreaking study titled “Neutron crystallography of a semiquinone radical intermediate of copper amine oxidase reveals a substrate-assisted conformational change of the peptidyl quinone cofactor,” researchers from Osaka Medical and Pharmaceutical University and Osaka University have utilized the advanced technique of neutron crystallography to overcome these limitations. This pioneering study offers unprecedented insights into the atom-by-atom structure of copper amine oxidase enzymes, shedding light on their biochemistry in a way that was previously unattainable.
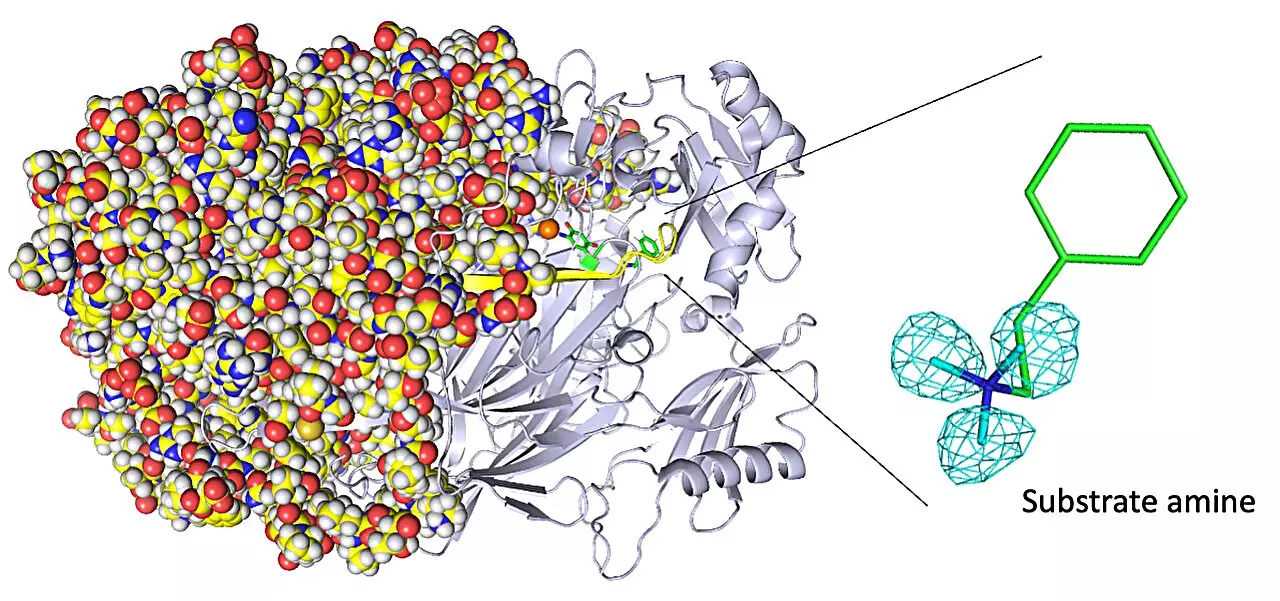
Copper amine oxidase enzymes exhibit fascinating biochemistry, including phenomena such as quantum tunneling that allows for remarkably fast reaction rates. Understanding the mechanisms behind these unique characteristics is crucial for designing artificial enzymes with similar capabilities. While X-ray crystallography is a widely used technique to determine enzyme structure, it falls short in imaging hydrogen atoms due to their minimal electron presence. This is where neutron crystallography comes into play, offering an alternative imaging technique that examines diffraction from atomic nuclei within the enzyme.
Through their experiments, the research team gained invaluable insights. One of their notable achievements was visualizing the protonation/deprotonation state, which is related to pH, of specific sites within the enzyme that play a crucial role in stabilizing radical species. Radical species are highly reactive atoms containing an unpaired electron. Additionally, the researchers observed and characterized the movements of the enzyme’s topaquinone cofactor, such as sliding, upward tilting, and half-rotation. These movements are essential for facilitating single-electron transfer within the enzyme, contributing to its overall functionality.
As a result of this study, the researchers revealed a previously unknown event occurring in the enzyme’s active site: the binding of a second molecule of high-affinity amine substrate during the enzymatic reaction. This insight, which X-ray crystallography failed to capture, adds to our understanding of the enzyme’s exceptional capabilities.
The intricate details uncovered through neutron crystallography allow for a more comprehensive comprehension of copper amine oxidase enzymes’ structural and functional features. This knowledge enables scientists to better explain why these enzymes exhibit remarkable efficiency at physiological temperatures and pressures. Furthermore, armed with these insights, researchers now have the potential to apply their findings to develop artificial enzymes for use in various biochemical and industrial applications.
The groundbreaking use of neutron crystallography in this study highlights the immense potential of this advanced imaging technique. By providing a detailed, atom-by-atom picture of enzyme structures, neutron crystallography revolutionizes our understanding of enzymatic biochemistry. With continued advancements in this area, scientists can unlock the mysteries of various enzymes, uncovering their intricate workings and empowering us to harness their potential for a wide range of applications.

Leave a Reply