Organic chemistry plays a pivotal role in various industries, including pharmaceuticals and chemicals. One class of organic compounds that holds great significance is heterocyclic compounds. These compounds consist of ring structures containing carbon atoms and other elements such as nitrogen, oxygen, or sulfur. Due to their versatility and excellent physiological activities, heterocyclic compounds have become highly sought after in the chemical and pharmaceutical industry. However, the current methods for synthesizing these compounds often involve high temperature, pressure conditions, or the use of precious metal catalysts, which contribute to the economic and environmental costs.
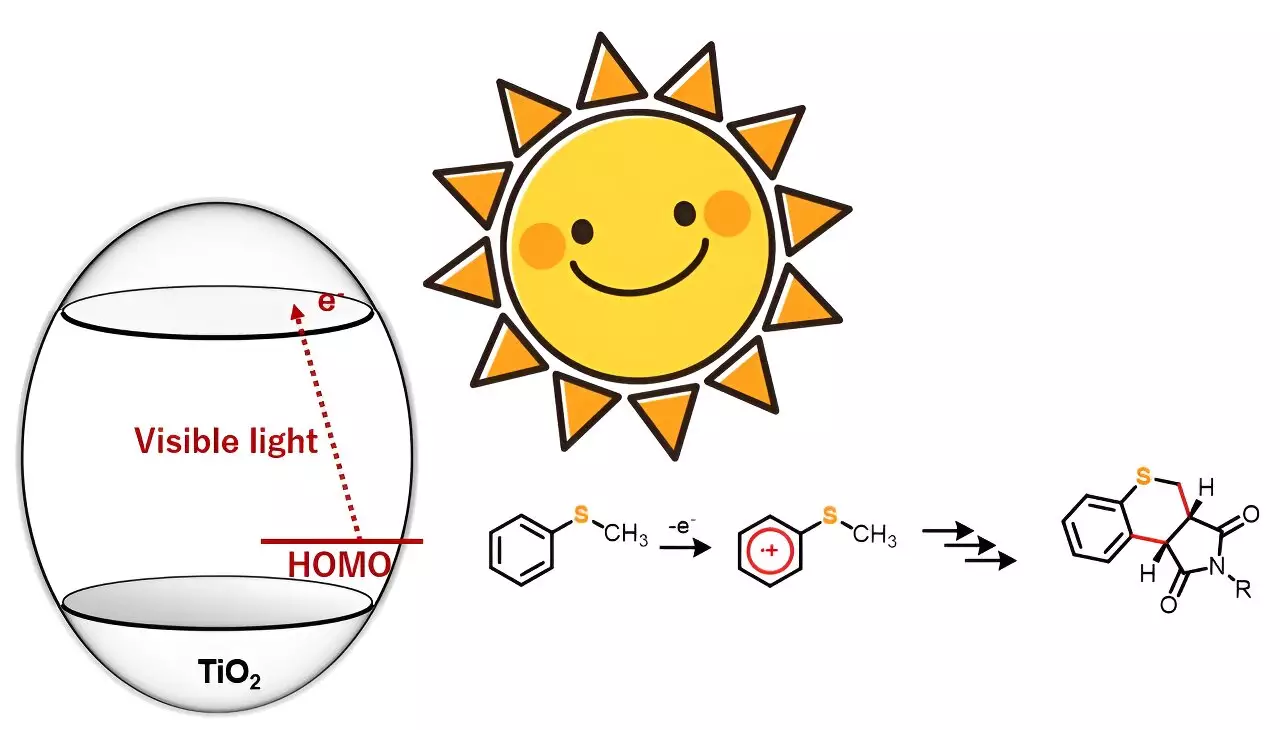
A recent study published in the journal Advanced Synthesis & Catalysis by a team of researchers from Japan and Bangladesh proposes a simple yet effective method for synthesizing heterocyclic compounds. Led by Professor Yutaka Hitomi from Doshisha University, the team demonstrated the synthesis of 20 sulfur-containing heterocyclic compounds using photocatalyst titanium dioxide (TiO2) and visible light. This breakthrough provides an alternative approach that addresses the challenges associated with traditional synthesis methods.
Photocatalyst titanium dioxide (TiO2) has gained attention among synthetic chemists for its potential in driving organic reactions. However, many existing processes require ultraviolet light to trigger the desired reactions. The research team discovered that under anaerobic conditions, sulfur-containing organic compounds combined with maleimide derivatives when exposed to blue light. This resulted in the formation of dual carbon-carbon bonds, leading to the synthesis of new heterocyclic organic compounds.
Unlike ultraviolet light, which generates highly oxidative holes, the team’s innovative approach allows for the selective one-electron oxidation of substrate molecules using visible light. This selective oxidation paves the way for various organic chemical reactions. Five 4-substituted thioanisoles and four N-substituted maleimides were chosen for annulation or ring formation reactions in this study. While no reaction occurred when the starting materials were exposed to blue light alone, the introduction of TiO2 into the reaction system enabled the synthesis of 20 different thiochromenopyrroledione derivatives with moderate-to-high yield.
The research team observed a substituent effect in the reactions to gain a deeper understanding of the corresponding mechanistic aspects. Based on the results, they postulated that the reaction proceeds through charge transfer from thioanisole to the conduction band of TiO2. Irradiation with blue light triggers one-electron oxidation of thioanisole, initiating the generation of α-thioalkyl radicals through deprotonation.
This study highlights the potential of TiO2 for visible light photocatalysis in organic synthesis. Not only does this offer a simplified and more environmentally-friendly approach compared to traditional methods, but it also provides important insights into the chemistry of complex heterocyclic compound synthesis. This groundbreaking research opens up new possibilities for transitioning from resource-intensive industrial chemical processes to a more energy-efficient system.
Professor Hitomi envisions that this visible light-driven technology could have a significant impact on the synthesis of pharmaceuticals. With the potential to make the synthesis process more accessible and affordable, this innovation could greatly improve the health and well-being of millions of people worldwide. The efforts of Professor Hitomi and his team represent a positive step towards the development of a sustainable chemical industry, paving the way for the revolutionization of multiple chemical industries.
The promising role of TiO2 in visible light photocatalysis for organic synthesis holds great potential for the future of chemical and pharmaceutical industries. By harnessing the power of visible light, the team of researchers from Japan and Bangladesh has provided a simplified and environmentally-friendly alternative to traditional synthesis methods. This breakthrough opens up new avenues for the synthesis of heterocyclic compounds, offering the potential to revolutionize multiple industries and contribute to a more sustainable future.

Leave a Reply