Water electrolysis, a process that produces hydrogen from water, holds great promise as an environmentally friendly technology. It is free from carbon dioxide emissions, making it an attractive avenue for hydrogen production. However, the reliance on precious metal catalysts, such as iridium (Ir), has hindered its economic feasibility. This limitation has prompted researchers to explore the development of catalysts in the form of metal alloys. In this pursuit, iridium, ruthenium (Ru), and osmium (Os) have emerged as the primary catalysts under scrutiny.
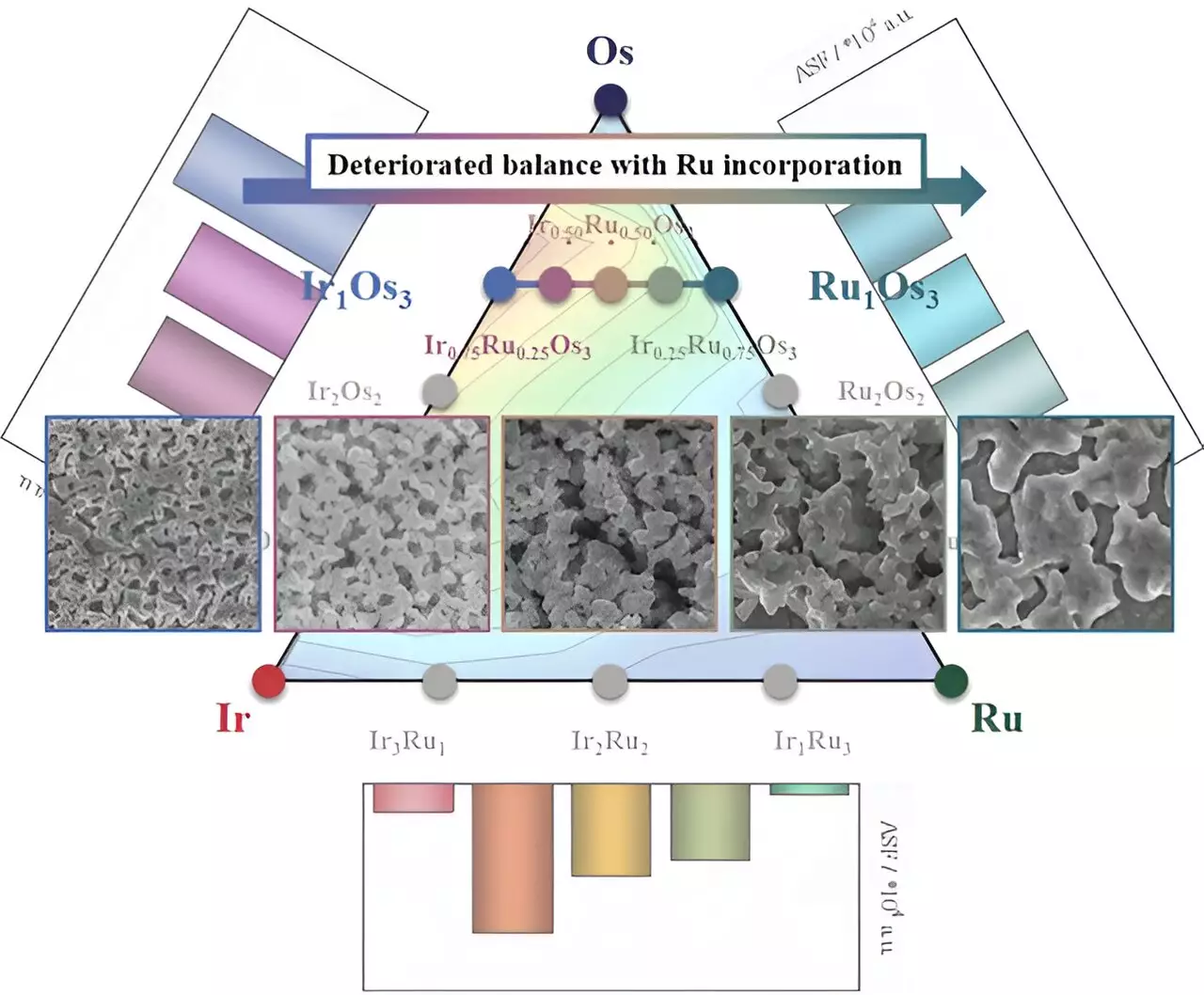
Iridium, known for its high stability, falls short in terms of activity and comes with a hefty price tag. On the other hand, ruthenium exhibits commendable activity and is more cost-effective than iridium. However, it lacks the same level of stability. Osmium, while promising, dissolves readily under various electrochemical conditions. Despite its tendency to dissolve, it leads to the formation of nanostructures with an expanded electrochemical active surface area, thereby enhancing geometrical activity.
Catalyst Development: Combining the Best of Iridium and Ruthenium
Professor Yong-Tae Kim and Kyu-Su Kim from Pohang University of Science and Technology (POSTECH) embarked on a research project to develop catalysts that address the limitations of water electrolysis. Initially, they focused on combining iridium and ruthenium to preserve the excellent attributes of each catalyst. This innovative approach proved successful, as the resulting catalysts displayed improvements in both activity and stability. By leveraging the strengths of iridium and ruthenium, the research team pushed the boundaries of water electrolysis catalysis.
Buoyed by their initial success, the research team incorporated osmium into the catalysts. Osmium demonstrated high activity due to the expanded electrochemical active surface area achieved through nanostructure formation. These catalysts retained the advantageous properties of both iridium and ruthenium. However, the dissolution of osmium had unforeseen consequences. It compromised the structural integrity of iridium and ruthenium, leading to the agglomeration and corrosion of nanostructures. This in turn resulted in a decline in the balance of catalytic performance.
Based on their findings, the research team proposes several avenues for further catalyst research. Firstly, they emphasize the need for a metric that can evaluate both activity and stability simultaneously. In 2017, Kim’s research group introduced the activity-stability factor, a metric that could prove instrumental in the development of superior catalysts. Additionally, the team advocates for the retention of superior catalyst properties even after the formation of nanostructures. This will enhance the electrochemical active surface area of the electrocatalyst, thus maximizing its performance. Lastly, they stress the importance of carefully selecting candidate materials that can effectively synergize when alloyed with other metals. This approach will facilitate the creation of catalysts with improved activity, stability, and overall performance.
The research led by Professor Yong-Tae Kim and Kyu-Su Kim explores the journey towards efficient and stable catalysts for hydrogen production through water electrolysis. By combining iridium and ruthenium, they achieved notable improvements in both activity and stability. The inclusion of osmium presented additional challenges but also demonstrated high activity. However, the dissolution of osmium compromised the structural integrity of the catalysts. Through their research, the team highlights the need for a comprehensive metric to evaluate catalyst performance, the importance of retaining superior properties in nanostructures, and the significance of careful material selection. While the study does not present specific outcomes, it provides essential considerations for future catalyst design. As researchers continue to unravel the mysteries surrounding catalysts, the quest for efficient and stable catalysts for hydrogen production marches on.

Leave a Reply