Single-use plastics are a pressing environmental concern, but researchers at the Institute for Chemical Reaction Design and Discovery (WPI-ICReDD) at Hokkaido University have discovered a groundbreaking method to repurpose plastic bags from grocery stores. Instead of ending up in landfills, these used plastic bags can be utilized to initiate chemical reactions that detoxify hazardous chemicals. This innovative approach not only enhances safety but also offers a practical solution for reusing common plastics while addressing the global plastic waste crisis.
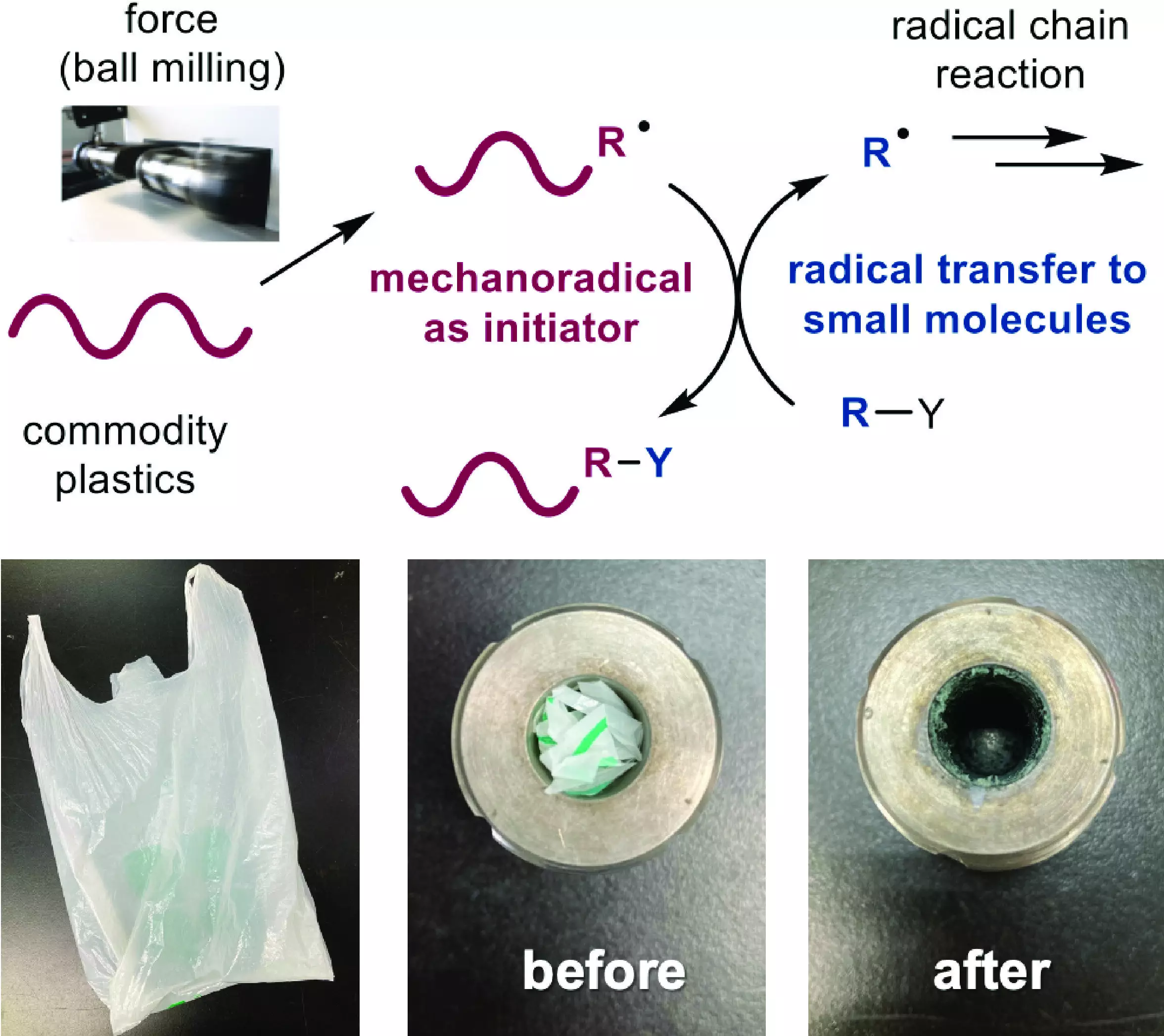
The team of researchers successfully developed a technique using common plastic materials, such as polyethylene and polyvinyl acetate, in place of potentially explosive compounds to initiate radical chain reactions. They utilized a ball mill, a machine that rapidly shakes a steel ball inside a steel jar to mix solid chemicals. The mechanical force generated by the ball breaks a chemical bond in the plastic, forming highly reactive radicals with unbonded electrons. These radicals then facilitate a self-sustaining chain reaction that promotes dehalogenation – the replacement of a halogen atom with a hydrogen atom – in organic halides.
A New Perspective on Organic Synthesis
Associate Professor Koji Kubota remarks, “The use of commodity plastics as chemical reagents is a completely new perspective on organic synthesis.” This groundbreaking approach not only enables the development of safe and highly efficient radical-based reactions but also provides a solution for the utilization of waste plastics, which are a severe social and environmental problem.
The research team showcased the reuse of waste plastic by adding plastic shreds from a common grocery bag to the ball mill jar. They successfully carried out the chemical reaction, illustrating the feasibility of utilizing plastic bags in this process. Furthermore, the researchers demonstrated that their method can effectively treat highly toxic polyhalogenated compounds commonly used in various industries. By employing polyethylene as an initiator for a radical reaction, they successfully removed multiple halogen atoms from a flame retardant compound, effectively reducing its toxicity.
The Potential for the Industry
The cost-effectiveness and safety advantages of this innovative method make it highly attractive to industries. Professor Hajime Ito suggests, “Our new approach using stable, cheap, and abundant plastic materials as initiators for radical chain reactions holds significant potential to foster the development of industrially attractive, safe, and highly efficient chemical processes.”
By repurposing used plastic bags for chemical reactions, researchers have introduced a groundbreaking solution to tackle the environmental issue of single-use plastics while simultaneously mitigating the hazards associated with chemical reactions. This new perspective on organic synthesis not only offers a way to safely and efficiently carry out radical-based reactions but also presents an opportunity to address the global challenge of plastic waste. With the potential to revolutionize industrial processes, this method has paved the way for a more sustainable and environmentally conscious future.

Leave a Reply