The COVID-19 pandemic has brought about unprecedented challenges for healthcare systems worldwide. As countries continue to battle the SARS-CoV-2 virus, researchers are tirelessly working to gain a better understanding of the virus and develop effective treatments. A recent study conducted by researchers at Kanazawa University, in collaboration with scientists from Chiba University, sheds light on the role of the open reading frame 6 (ORF6) protein in COVID-19 symptoms. Utilizing high-speed atomic force microscopy (HS-AFM), the researchers explored the structure and behavior of ORF6, providing valuable insights into its potential therapeutic targets.
SARS-CoV-2, like many other viruses, produces various accessory proteins to manipulate the host environment and promote its replication. Among these accessory proteins is ORF6, which has been implicated in interfering with the function of interferon 1 (IFN-I), a vital component of the immune system. Previous studies have suggested that the disruption of IFN-I signaling by ORF6 may contribute to asymptomatic infections. However, the exact mechanism behind this interference and its impact on COVID-19 symptoms remained unclear.
Unraveling the Structure of ORF6
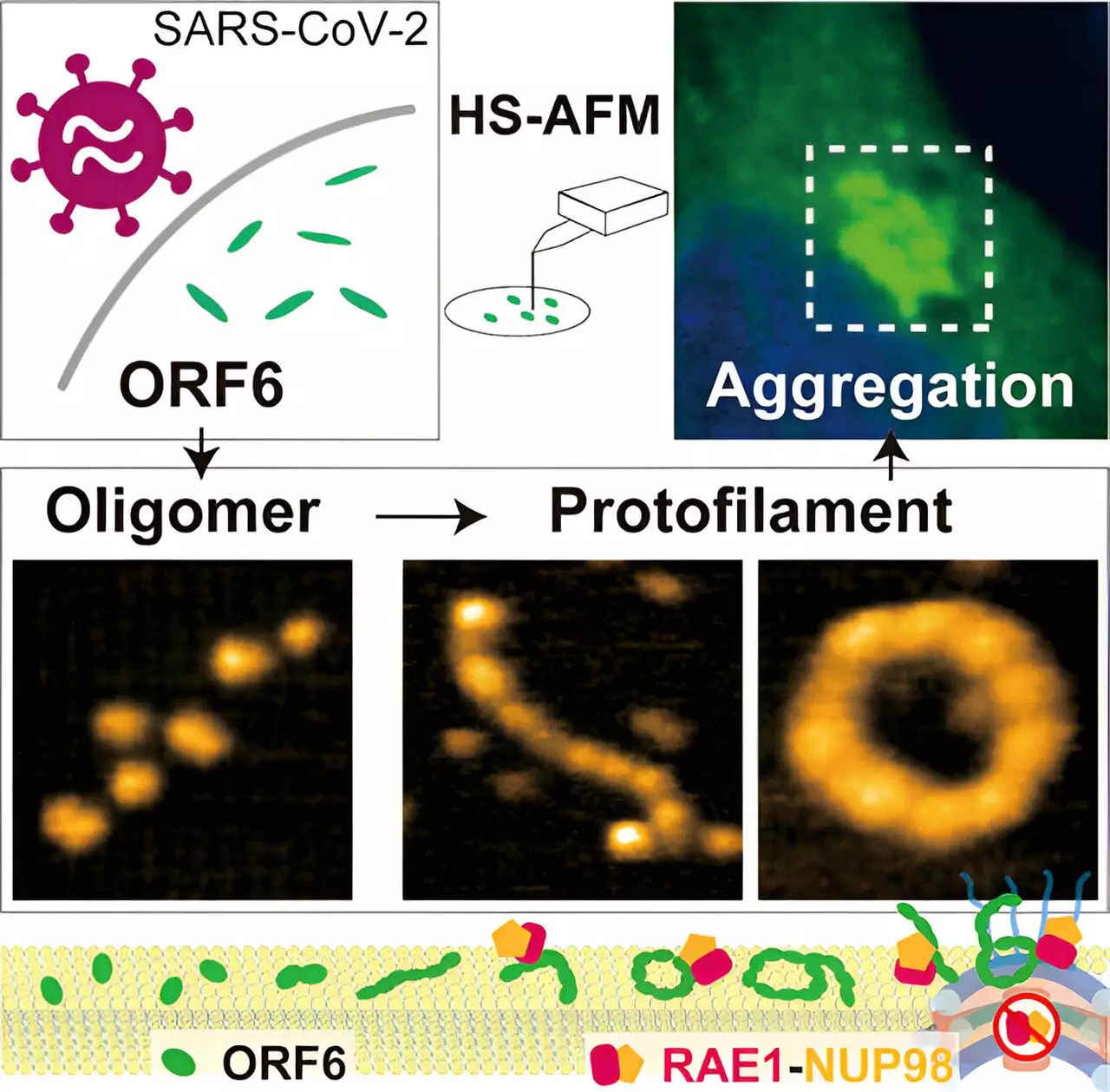
To gain insights into the structure and behavior of ORF6, the researchers employed HS-AFM. Unlike traditional methods, HS-AFM enabled them to capture the topography and dynamics of ORF6 with high precision and speed. Utilizing software programs and nuclear magnetic resonance measurements, the researchers identified the presence of intrinsically disordered regions in ORF6. These findings posed challenges for using machine learning algorithms like AlphaFold2 to establish the protein’s structure, thus necessitating the use of HS-AFM.
The HS-AFM analysis revealed that ORF6 primarily exists in the form of ellipsoidal filaments of oligomers. These filaments consist of repeating molecular units but are shorter than polymers. Interestingly, the length and circumference of the filaments varied depending on the temperature, with the largest filaments observed at 37°C and the smallest at 4°C. This observation implies that the presence of fever in COVID-19 patients could facilitate the formation of larger filaments, potentially impacting disease progression.
Additionally, the researchers found that lipid substrates promoted the formation of larger ORF6 oligomers. This suggests that the interaction between the protein and fatty compounds may contribute to its structural organization. Furthermore, the HS-AFM analysis allowed for the observation of protein dynamics, including circular motion, protein assembly, and flipping. These dynamic behaviors provide valuable insights into the behavior of ORF6 within the cellular environment.
Aggregate Formation and COVID-19 Complications
Computer analysis revealed that the ORF6 filaments had a tendency to aggregate into amyloids, similar to those found in certain neurodegenerative diseases. This aggregation plays a crucial role in sequestering host proteins, particularly transcription factors involved in IFN-I signaling. The disruption of IFN-I signaling can have significant consequences for the immune response to viral infections, potentially resulting in severe COVID-19 symptoms. Understanding the aggregation behavior of ORF6 opens up new avenues for the development of targeted therapeutic interventions.
Potential Therapeutic Targets
The researchers’ findings suggest that the ORF6 protein is mainly held together by hydrophobic interactions. This insight provides potential therapeutic targets for the management and treatment of COVID-19. Compounds that can disrupt hydrophobic interactions and dissociate ORF6 aggregates should be investigated for their therapeutic value. Identifying druggable candidates that can effectively target and disrupt the aggregation of ORF6 may lead to the development of novel treatments for COVID-19.
The utilization of high-speed atomic force microscopy has allowed researchers to gain crucial insights into the structure, behavior, and potential therapeutic targets of the ORF6 protein in COVID-19 symptoms. By understanding the mechanisms by which the virus manipulates host proteins, we can develop more effective treatments and strategies to combat the spread of the SARS-CoV-2 virus. Further research is essential to validate these findings and pave the way for the development of targeted therapies to mitigate the impact of COVID-19.

Leave a Reply