The discovery of a unique class of anticancer molecules in a family of bryozoans nearly 30 years ago opened up a new frontier in the field of organic chemistry. These molecules, characterized by their dense, highly complex structures composed of oxidized rings and nitrogen atoms, have long fascinated scientists and posed a significant challenge in terms of synthesis. Despite numerous attempts by research groups worldwide, recreating these intricate molecules in the laboratory has remained an elusive goal.
Numerous research groups have attempted to recreate the anticancer molecules found in bryozoans, but the dense and intricately connected nature of their structures has made it nearly impossible. The highly reactive nature of certain heterocyclic rings, such as the indole ring, has further complicated the synthesis process, leading to a series of failed attempts over the past few decades. However, a recent breakthrough by a team of Yale chemists has changed the game entirely.
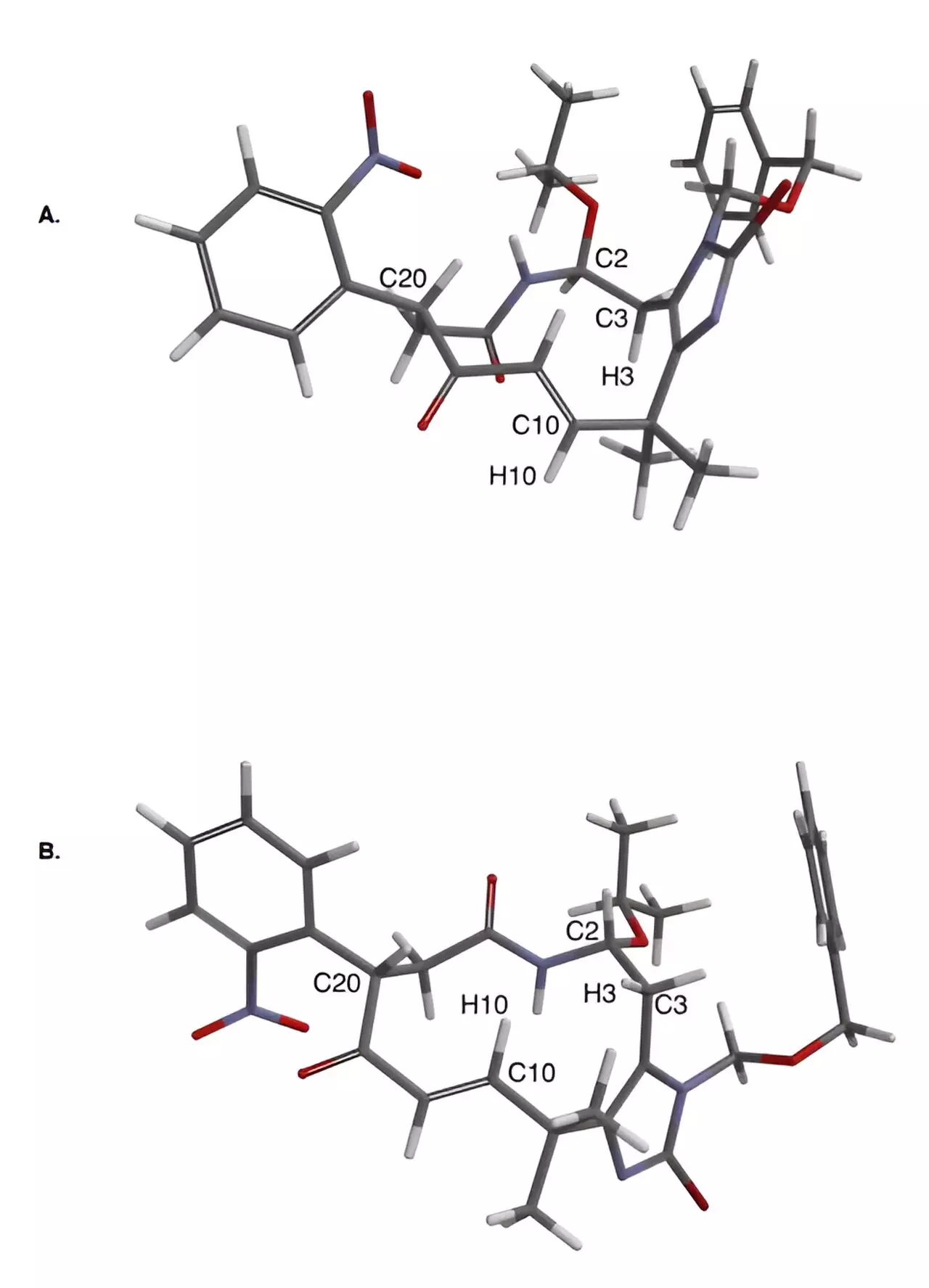
The Yale chemists utilized a novel approach that combined inventive chemical strategies with cutting-edge technology in small molecule structure determination to successfully synthesize eight of the compounds for the first time. One key strategic element of their approach was avoiding the construction of the reactive heterocyclic ring until the end of the process, thus minimizing potential issues. Additionally, they employed oxidative photocyclizations to construct crucial bonds in the molecules, leveraging methods first studied at Yale in the 1960s. Finally, the use of microcrystal electron diffraction (MicroED) analysis helped visualize the structures of the molecules, overcoming limitations of traditional structure determination methods.
The result of this groundbreaking approach is the creation of eight new synthetic molecules with promising therapeutic potential, marking a significant advancement in the field of synthetic chemistry. These molecules, derived from a specific species of bryozoa called Securiflustra securifrons, have the potential to serve as novel anticancer agents and pave the way for further discoveries in medicinal chemistry. The success of this research would not have been possible without the dedication and expertise of the team, including co-first authors Brandon Alexander and Noah Bartfield, along with other members who contributed to the study.
The synthesis of these anticancer molecules represents a major milestone in the field of organic chemistry and opens up exciting possibilities for the development of new medications. The innovative strategies and technologies employed by the Yale chemists have shed light on previously unexplored pathways for synthesizing complex molecules, demonstrating the power of interdisciplinary collaboration and scientific perseverance in overcoming seemingly insurmountable challenges in chemistry.

Leave a Reply