The behavior of free electrons in water has been a topic of discussion in the field of physics for quite some time. When water comes into contact with radiation, free electrons are released from the water molecules as they ionize. However, the flow and behavior of these electrons between water molecules has remained a contentious issue. In an effort to gain a better understanding of this phenomenon, a team of theoretical physicists at DESY collaborated with experimental scientists from Argonne National Laboratory to investigate the presence of free electrons in water at very short time scales.
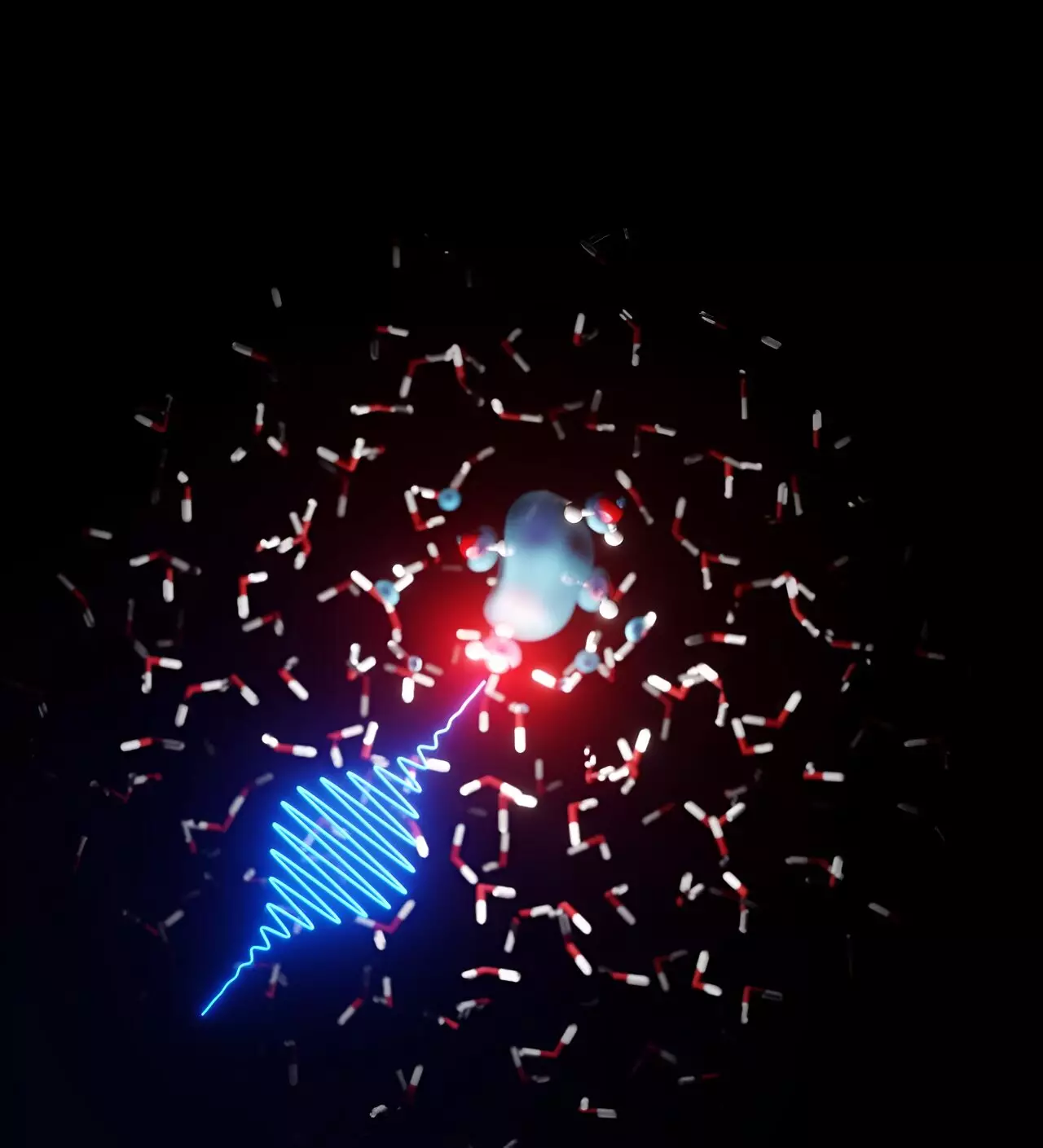
Using data obtained from the LCLS X-ray laser at the SLAC National Accelerator Laboratory in California, the experimental team led by Linda Young observed peculiar signatures associated with water molecules that were excited by lasers and imaged by the X-ray laser. Through X-ray absorption spectroscopy, they identified structures among the water molecules. To further analyze these findings, the experiment team enlisted the expertise of theoretical physicists at DESY.
Under the leadership of DESY scientist Ludger Inhester, the theoretical physics team worked with the experimental data to develop models. Their collaborative efforts resulted in the revelation that free electrons in water form bubble structures that are then encased within water molecules, analogous to the solvation of chemicals at the molecular level. The DESY team was able to decipher the process and parameters behind this solvation of electrons in water.
One key insight that emerged from their research is the remarkable sensitivity of the solvation process to temperature changes in the water. The dissolution process and subsequent formation of cage-like structures were found to be intricately linked to variations in water temperature. Arturo Sopena, the first author of the study, emphasized the substantial impact that temperature fluctuations can have on the electron solvation process.
The findings also shed light on the rapidity of the electron solvation process. Initially, the electron can be found dispersed across a wide area among the water molecules. However, it quickly docks onto specific hydrogen bonding patterns within the molecular liquid water, leading to a deep “burrowing” within a very narrow region of the water structure. This reorientation of neighboring water molecules takes place within a mere 100 femtoseconds, while the disassociation of the bubble structure occurs within several picoseconds.
Understanding how water reacts when exposed to radiation is of utmost importance given its relevance to both chemical and biological systems. The newly discovered chemical reaction steps driven by radiation play a crucial role in determining subsequent radiation chemistry, which applies not only to inanimate materials but also to biological substances. The insights gained from this research contribute to a deeper comprehension of radiation damage caused by ionizing radiation in water.
The research conducted by the team from DESY and Argonne National Laboratory was also part of the Cluster of Excellence CUI: Advanced Imaging of Matter at Universität Hamburg. Building on these findings, the establishment of the Center for Molecular Water Science at DESY aims to intensify water-related research in collaboration with international partners.
The study conducted by the theoretical physicists at DESY and the experimental scientists at Argonne National Laboratory has shed new light on the behavior of free electrons in water. Through their collaborative efforts, they have revealed the formation of bubble structures encased within water molecules during electron solvation. The sensitivity to temperature changes, rapidity of the solvation process, and implications for radiation chemistry further enhance our understanding of the complex interplay between radiation and water. These findings pave the way for future research in the field of molecular water science, ultimately advancing our knowledge of the fundamental processes occurring in biological and chemical systems.

Leave a Reply