In the quest for sustainable energy solutions, researchers are turning their sights toward ammonia, a compound not only vital for food production but also emerging as a potential zero-carbon fuel. The global food supply chain relies heavily on ammonia as it’s an essential ingredient in fertilizers. However, the traditional method of ammonia production, known as the Haber-Bosch process, is notoriously inefficient and contributes significantly to greenhouse gas emissions—accounting for approximately 1.8% of global CO2 outputs. This alarming statistic has created an urgent demand for alternative approaches that minimize environmental impact while maintaining energy efficiency. Recent advancements in the electrochemical nitrate reduction reaction (eNO3RR) have showcased promise in this quest, providing a potential route to produce ammonia sustainably from nitrate waste.
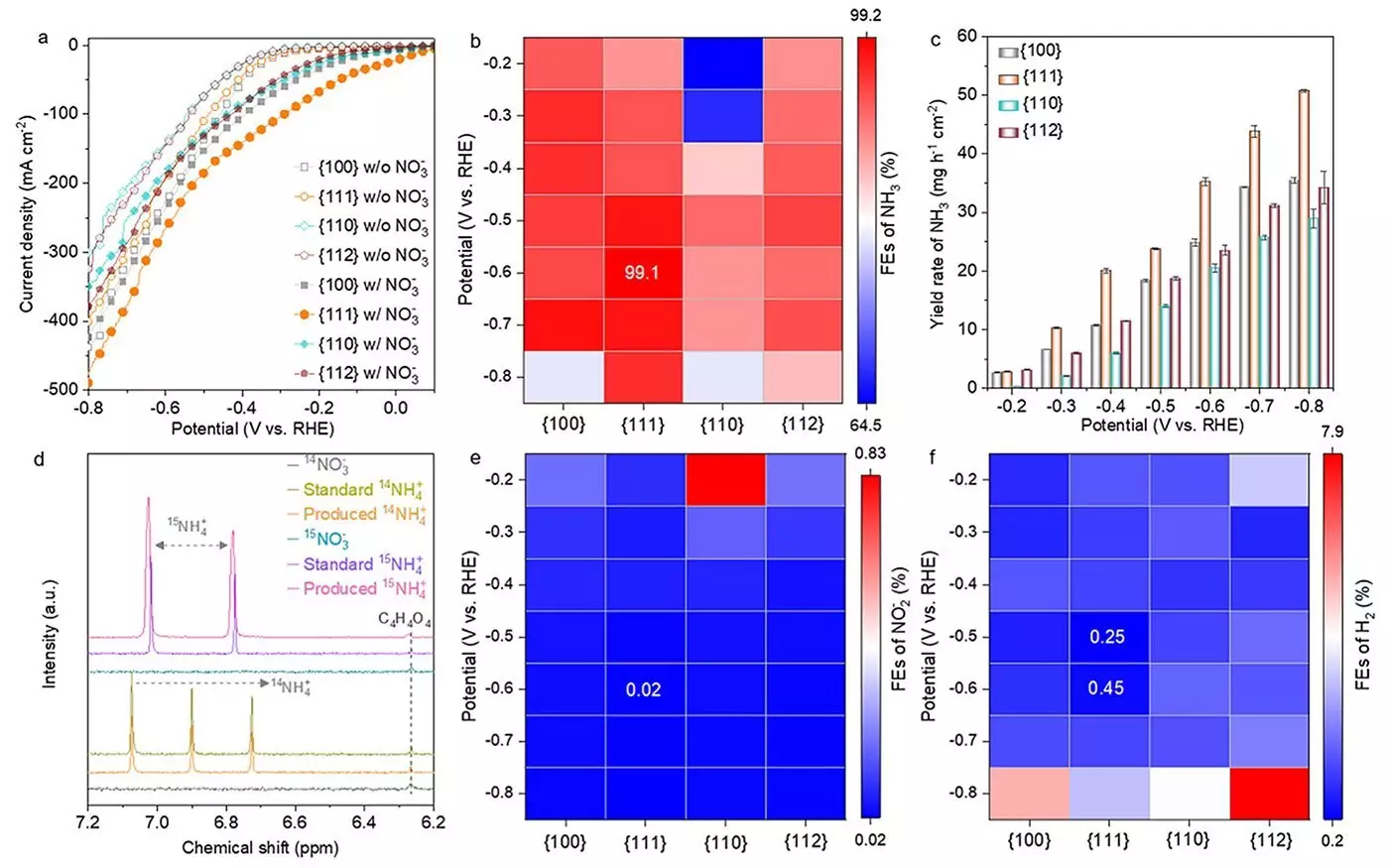
A pivotal aspect of eNO3RR is the search for efficient catalysts that can facilitate the conversion of nitrate into ammonia effectively. A recent study published in ACS Nano highlights advances in the use of cobalt oxides, specifically spinel cobalt oxides (Co3O4), which have emerged as frontrunners due to their cost-effectiveness and high catalytic activity. This research delves into various Co3O4 nanostructures, focusing particularly on their crystallographic facets—namely, {100}, {110}, {111}, and {112}—to discern how these structural differences impact their catalytic performance in ammonia production.
The research findings revealed that the {111} facet of Co3O4 demonstrated exceptional performance, achieving an ammonia Faradaic efficiency of an astonishing 99.1%. In practical terms, this translates to a remarkable yield rate of 35.2 mg h-1 cm-2, illustrating the importance of facet orientation in managing catalytic efficiency. The success of the {111} facet can be attributed to the rapid creation of oxygen vacancies and the development of cobalt hydroxide (Co(OH)2), processes that significantly enhance the catalyst’s reactivity.
The transformation that the catalyst undergoes during ammonia production highlights another layer of complexity in its performance. Researchers observed that Co3O4 doesn’t merely function as a stable catalyst but undergoes a series of structural changes throughout the reaction process. Initially, Co3O4 transitions through states with oxygen vacancies before finally stabilizing as a Co(OH)2 hybrid. This evolution underscores the dynamic nature of the catalyst and its relationship with the reaction environment.
These insights into the catalyst’s transformation are crucial, as Dr. Heng Liu, a key contributor to the study, emphasizes. Understanding how structural changes impact catalytic activity can ultimately guide the design of improved catalysts, optimizing them for efficiency and sustainability. This aligns with the broader objective of enhancing the industrial application of catalysts in green chemistry.
The implications of this research extend beyond ammonia production and into the realms of renewable energy and environmental sustainability. By transforming nitrate waste—a common pollutant in agricultural runoff—into valuable ammonia, the eNO3RR process not only mitigates pollution but also supports sustainable ammonia production that could replace the Haber-Bosch method. This dual benefit presents a significant opportunity for industry players seeking to align with carbon neutrality goals set for the coming decades.
Additionally, ammonia holds potential as a clean fuel source. Its properties, including high energy density and clean combustion, position it as a candidate for future energy technologies. As the world grapples with the pressing need for innovative solutions to combat climate change, the development of efficient catalysts for sustainable ammonia production is both timely and essential.
Looking ahead, researchers aim to refine the catalytic process further by controlling the final stages of catalyst transformation. Such improvements could lead to enhanced activity, selectivity, and stability of the catalysts used in eNO3RR, ultimately paving the way for cleaner and more sustainable manufacturing processes. As Professor Hao Li articulates, these advancements could significantly contribute to the global goal of achieving carbon neutrality by the 2050s, reinforcing the vital role of research and innovation in the face of environmental challenges.
The progress made in understanding and optimizing Co3O4 catalysts marks a significant step toward sustainable ammonia production and underscores the importance of interdisciplinary research in addressing global energy and environmental issues. The future of ammonia as a clean energy source hinges on further developments in this exciting field of study.

Leave a Reply