Noble gases have long been characterized as the wallflowers of the periodic table: rarefied, seemingly inert substances that resist interaction with other elements. However, the groundbreaking work of chemist Neil Bartlett over 60 years ago shattered this conventional wisdom. By synthesizing the first noble gas compound, xenon hexafluoroplatinate (XePtF6), Bartlett’s experiment opened the door to the study of noble gas compounds, although the complexities involved in growing suitable crystals continued to challenge researchers for decades. This initial discovery laid the foundation for what has since become a vibrant field of exploration, sparking enthusiasm among scientists in the quest to understand the nuances of these enigmatic elements.
The elusive nature of noble gas compounds stems primarily from the instability of their crystal forms. Sensitive to moisture and environmental conditions, growing large enough crystals for detailed analysis has proven to be a daunting task. Traditionally, researchers have relied on single-crystal X-ray diffraction for structural characterization. However, this method’s inherent limitations have meant that many potentially rich compounds, including Bartlett’s XePtF6, remain inadequately characterized. The delicate chemistry underlying noble gas compounds necessitates specialized techniques and procedures that have yet to be universally applied, hindering the pace of discovery in this field.
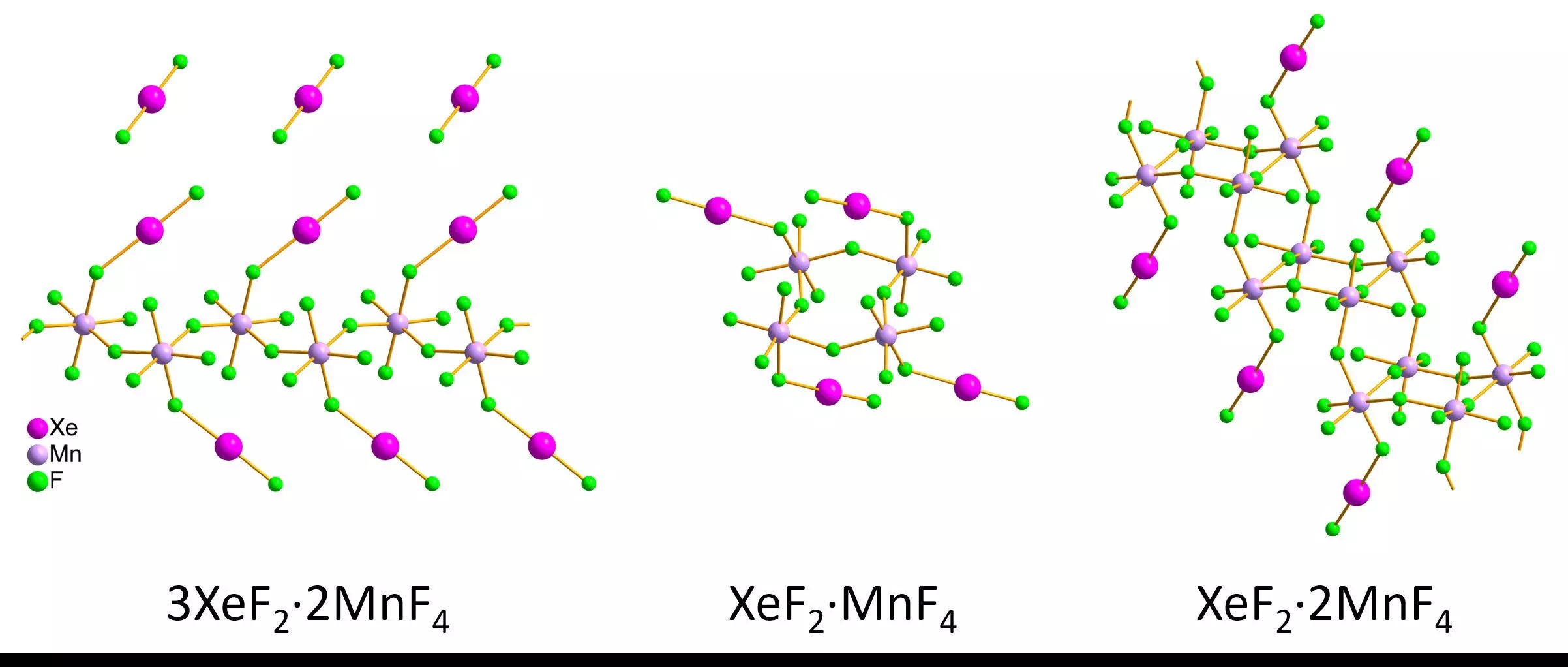
Recent advancements in analytical technology have spurred renewed interest in examining noble gas compounds. A new technique known as 3D electron diffraction has emerged as a game-changer, allowing researchers to examine nanoscale crystallites that display stability in air. Pioneering studies, such as those conducted by Lukáš Palatinus and Matic Lozinšek, involved synthesizing xenon difluoride-manganese tetrafluoride compounds. By cooling the sample holder with liquid nitrogen and establishing protective layers during the transfer to electron microscopes, the research team successfully measured bond lengths and angles in their nanocrystalline samples.
The critical breakthrough in this research was the ability to compare results obtained through 3D electron diffraction with larger, traditional crystal forms analyzed by single-crystal X-ray diffraction. Despite some minor discrepancies, the agreement between the two techniques affirmed the validity of both methods. The structures identified—to include infinite zig-zag chains, rings, and staircase-like double chains—reflect the intricate arrangements possible in xenon compounds. This dual validated approach allows scientists to increasingly map the structural landscape of noble gas compounds and paves the way for characterizing others that have been lost to time.
With the successful utilization of 3D electron diffraction on xenon compounds, researchers are optimistic that this method can yield insights into XePtF6 and other noble gas compounds previously thought to be beyond reach. The challenges presented by air-sensitive substances may soon be mitigated, ensuring that the exploration of noble gases continues to be a frontier brimming with possibilities. As technological innovations further refine these methods, the potential for groundbreaking discoveries into the reactivity and properties of noble gases are brighter than ever, fueling excitement within the scientific community.

Leave a Reply