Bacteria have long been a formidable adversary in the battle against infectious diseases. These microorganisms have evolved a variety of protective strategies to survive hostile environments, evade host immune responses, and persist despite medical interventions. A critical area of research focuses on the structural elements that allow pathogenic bacteria to fortify themselves, particularly through the use of protective sugar-coated shells known as capsular polymers. Understanding the complexities of these capsules opens new doors for drug development and vaccine production.
The capsular polymers serve as a primary defensive mechanism for many bacterial pathogens. By enveloping themselves in these carbohydrate-rich shells, bacteria are not only shielded from physical stressors and desiccation, but also rendered invisible to the host’s immune system. This invisibility is crucial; an intact capsule can thwart immune attack, essentially buying the bacteria time to multiply and potentially cause illness. Without this protective layer, pathogenic bacteria could be significantly weakened, suggesting that targeting the capsule formation process may offer a viable avenue for therapeutic interventions.
This understanding has led researchers to focus on the enzymes responsible for synthesizing these capsules. Such enzymes are promising candidates for the development of new antibacterial agents and vaccines, starkly illustrating the intersection of basic research and applied biotechnology. While the importance of the capsules is well recognized, the specific mechanisms of how these polymers connect to the bacterial membrane remain largely elusive.
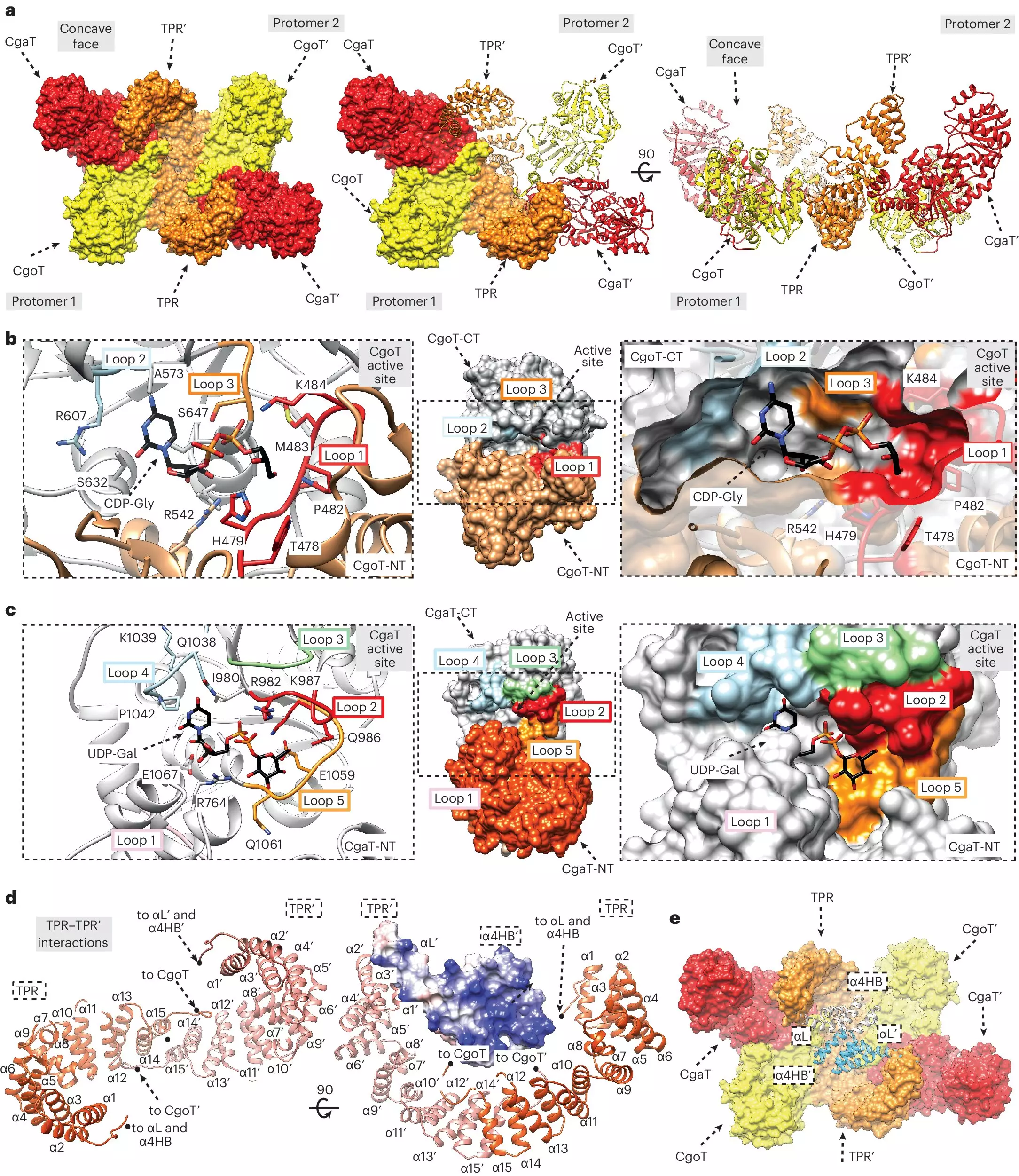
Recent research efforts, particularly those led by Dr. Timm Fiebig and his team at Hannover Medical School, have made significant strides in elucidating the molecular architecture that supports capsule development. The work revolves around identifying a crucial intermediary known as the “linker,” which acts as a connector between the lipid membrane—the cell’s anchor—and the outer capsules themselves. This discovery is pivotal; it provides insight into how various bacteria maintain their protective envelopes and could lead to the development of targeted antibacterial therapies.
Dr. Fiebig and his collaborators have characterized not only the linker but also the transition transferases, a group of enzymes that facilitate its production. These enzymes are integral to the overall biosynthetic pathway for capsule formation. Their research demonstrates that the unique interactions between the linker and the polymerases, the enzymes that construct the capsule’s sugar chains, can significantly affect the capsule’s functionality and, by extension, the bacteria’s virulence.
Understanding the role of transition transferases in designing the capsule opens the door to potential therapeutic strategies that could disrupt these protective mechanisms. Dr. Litschko’s findings that transition transferases are conserved across different bacterial genomes indicate a uniformity that might be exploited to create broad-spectrum antibiotics. By disrupting the enzymes responsible for synthesizing the linker, researchers could hinder the bacteria’s ability to form their protective capsules. This would make them more vulnerable to the immune system and could decrease the incidence and severity of infections caused by encapsulated pathogens.
Notably, the implications extend beyond immediate therapeutic applications. The ability to synthesize and analyze these linker-related enzymes in vitro—the test tube studies conducted by the research team—opens avenues for developing vaccines that leverage capsular components as immunogens. The insights gained through their research could facilitate the creation of vaccines that not only bolster immunity against specific pathogens but also against multiple strains, enhancing public health outcomes.
The ongoing research is not merely an academic exercise; it carries substantial public health implications. The challenge of antibiotic resistance looms large, making the discovery of novel drug targets all the more urgent. By pinpointing the mechanisms that support bacterial survival, researchers like Dr. Fiebig are paving the way for innovative strategies to counteract resistant strains and to enhance the effectiveness of existing treatments.
Moreover, the study’s findings regarding the structural differences between linker elements and capsular polymers serve as a testament to the complexity of microbial life and its adaptive capabilities. Each new discovery in this field contributes to a broader understanding of bacterial biology, ultimately aiding in the design of more effective countermeasures against infectious diseases.
As our understanding of bacterial protective mechanisms deepens, we find ourselves better equipped to combat the persistent threat these microorganisms pose. Research endeavors focusing on the intricacies of capsular biosynthesis not only enrich our scientific knowledge but also lay the groundwork for transformative medical advancements. It is crucial that we continue to explore these avenues to ensure a healthier future.

Leave a Reply