Aluminum oxide, known chemically as Al2O3, has long been recognized not just for its practical applications—ranging from electronic insulators to catalysts—but also for its complex structural properties. Often referred to by its mineral names like corundum, sapphire, or ruby, alumina is a quintessential material studied in various scientific fields. This article explores the recent advancements by researchers at TU Wien and the University of Vienna, who have illuminated the long-standing enigma surrounding the surface structure of aluminum oxide.
Understanding how chemical reactions occur on the surfaces of substances is crucial in fields such as catalysis and material science. In the case of aluminum oxide, the arrangement of surface atoms significantly influences its reactivity and performance in different applications. Traditionally, the structure of the surface has remained elusive, as thorough experimental analysis was hindered by the material’s high insulating properties. The difficulty in accurately determining this surface structure has persisted for decades, stalling advancements in both academic research and practical applications.
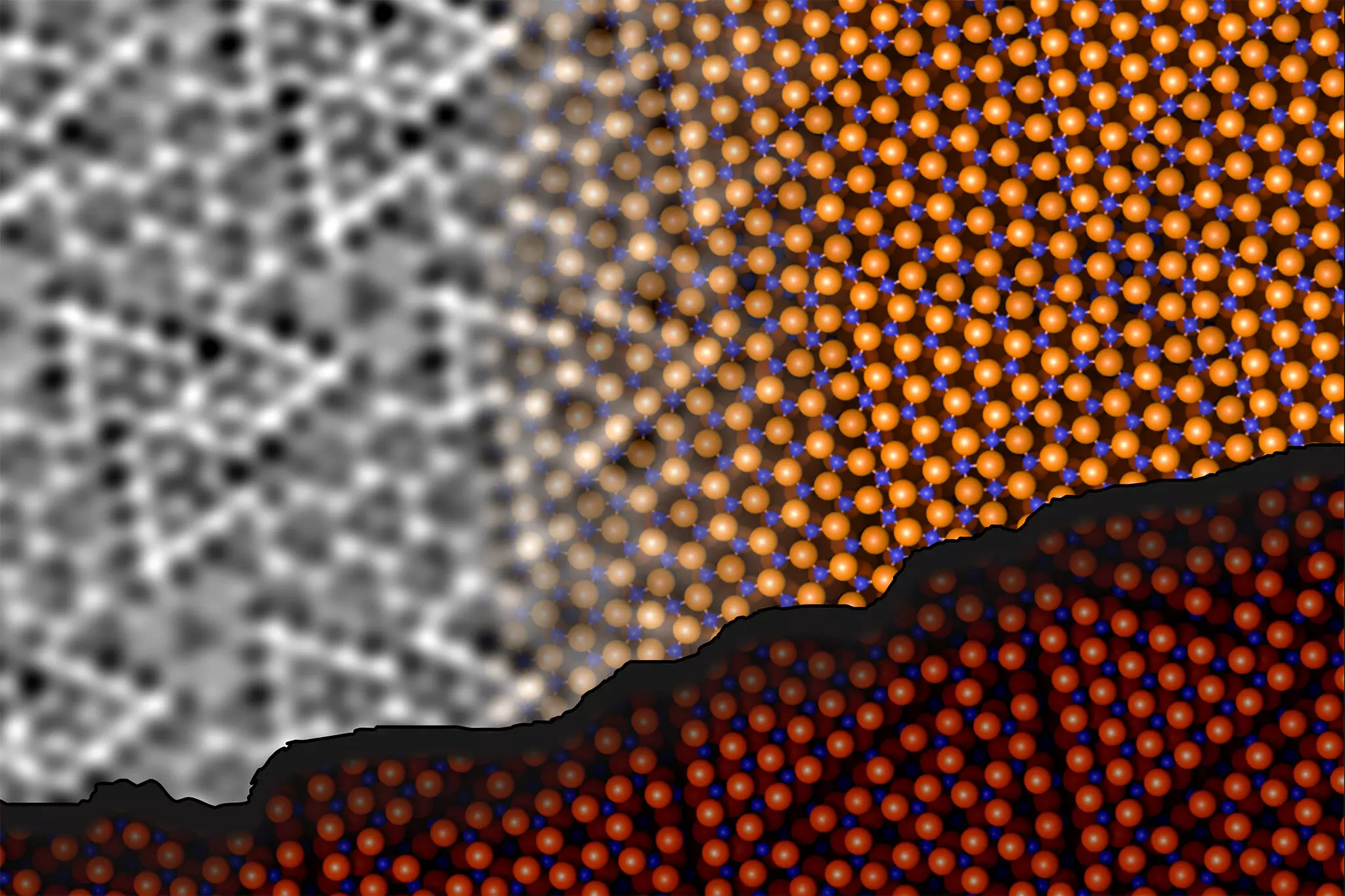
The researchers led by Jan Balajka and Ulrike Diebold recently employed innovative techniques to analyze the aluminum oxide surface using noncontact atomic force microscopy (ncAFM). This cutting-edge method involves scanning a sharp tip near the material’s surface without ever making contact, allowing for atomic-level imaging while avoiding any potential damage to the delicate surface. The frequency alterations of a quartz tuning fork provide insights into the interactions occurring at the atomic level, despite the method not being inherently chemically sensitive.
By modifying the scanning tip to incorporate a single oxygen atom, the researchers achieved a critical advancement; they were able to differentiate between oxygen and aluminum atoms. As Johanna Hütner explains, the carefully controlled tip allows for visual mapping of repulsion and attraction forces at the surface, offering insights into not just the locations of atoms but also their identities.
One of the major revelations from this research is the surface atoms’ ability to rearrange themselves. The team discovered that aluminum atoms at the surface could penetrate deeper into the material and form bonds with oxygen atoms situated in the inner layers. This dynamic rearrangement does not alter the fundamental numerical ratio of aluminum to oxygen but instead enhances structural stability by reducing energy levels.
Such insights challenge previously held beliefs and underscore the complexities inherent in the surface chemistry of aluminum oxide. Understanding these relationships is instrumental for scientists to manipulate and improve the performance of alumina in various applications, particularly in catalysis, where surface interactions play a vital role.
In navigating the complexities of the aluminum oxide surface, the research team incorporated machine learning techniques to optimize a three-dimensional model of the material. The intricate arrangement of atoms poses a significant challenge for traditional modeling methods, necessitating advanced algorithms to sift through a vast possibility space. Andrea Conti, who engaged in this computational work, highlighted the collaboration between experimental and computational techniques as a key element in solving this long-standing puzzle.
Machine learning not only facilitated the modeling process but also revealed structural design principles that could be applicable to a broader range of inorganic materials. The ability to predict and visualize complex interactions paves the way for future innovations in material development.
The findings discussed, detailed in their publication in *Science*, are influential not only for understanding aluminum oxide but also for creating pathways toward new material designs that could impact catalysis, energy storage, and even electronic device manufacturing. By elucidating the structure of this enigmatic insulator, the research opens up new avenues for experimentation and application, signaling a promising horizon for both material science and surface chemistry.
The recent breakthroughs by the research team exemplify the synergy of modern technology and classic scientific inquiry. The detailed exploration of aluminum oxide’s surface structure marks a pivotal milestone that could lead to transformative approaches in multiple high-tech industries, enhancing both efficiency and effectiveness across the board.

Leave a Reply