The rising levels of carbon dioxide (CO2) in the atmosphere pose significant environmental challenges, leading to increased scrutiny on methods for CO2 utilization. Among various strategies, the electrochemical reduction of CO2 has been recognized for its potential to transform this greenhouse gas into useful products. Traditionally, research has focused on optimizing catalysts through design modifications. However, the influence of electrolyte composition—a critical variable—has remained under-explored, leading to a significant gap in our understanding of how to manipulate product selectivity in CO2 reduction processes.
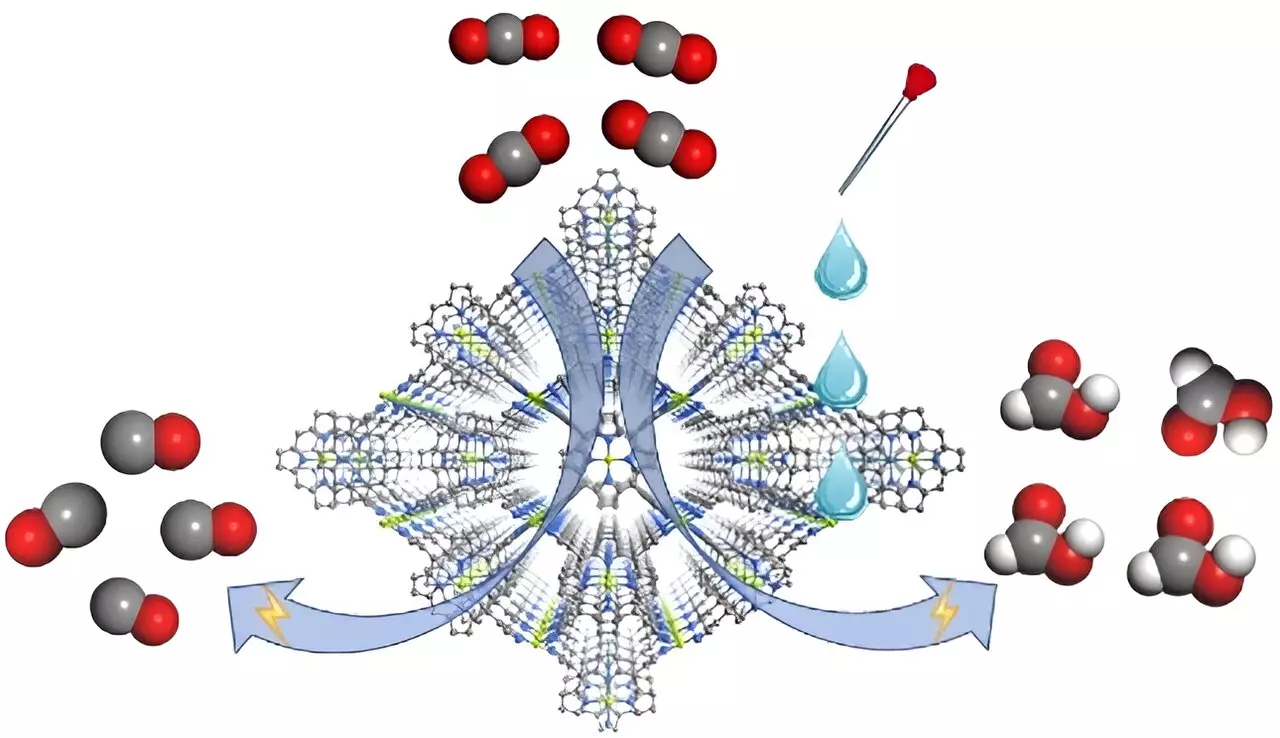
Recent research featured in Angewandte Chemie International Edition highlights a novel advancement in this field with the introduction of a metal-organic framework (MOF) electrocatalyst, named FICN-8. Developed by a team led by Prof. Cao Rong and Prof. Zhang Teng from the Fujian Institute of Research on the Structure of Matter, FICN-8 exemplifies a sophisticated approach to CO2 reduction that effectively integrates electrolyte composition. This MOF is crafted from ligands derived from copper porphyrin and building units composed of copper pyrazolate, forming a three-dimensional porous structure that enhances access to catalytic sites.
Electrochemical testing of FICN-8 revealed notable activity levels for CO2 reduction. In a specific electrolyte composed of tetrabutylammonium hexafluorophosphate (TBAPF6) diluted in acetonitrile, the catalyst demonstrated an impressive 95% selectivity towards carbon monoxide (CO). However, a transformative result emerged when proton sources such as water or trifluoroethanol (TFE) were introduced into the electrolyte. The product profile transitioned from predominantly CO to formic acid, illustrating the catalyst’s remarkable adaptability. The peak Faradaic efficiency for formic acid production reached 48% with the appropriate concentrations of water or TFE, underlining the vital role of the electrolyte in directing reaction pathways.
To further unravel the nuances of product selectivity, the researchers conducted kinetic isotope effect (KIE) measurements. These experiments provided critical insights, showing a consistent ratio for CO production alongside a significantly higher KIE value of 3.7 for formic acid synthesis, suggesting that the formation of HCOOH is intricately tied to proton participation. Theoretical analyses confirmed that the adsorption of hydride (*H) on specific nitrogen sites in the porphyrin plays a pivotal role in HCOOH formation, contrasting the CO production pathway, which operates independently of proton concentration.
This groundbreaking study emphasizes the often-overlooked influence of electrolyte composition on the selectivity of electrochemical CO2 reduction products. The findings advocate for a paradigm shift towards the development of innovative catalyst-electrolyte systems aimed at optimizing the production of valuable chemicals from CO2. As researchers continue to explore this domain, such advancements not only contribute to addressing climate change challenges but also pave the way for sustainable energy solutions. The intricate dance between catalyst design and electrolyte composition opens new avenues for scientific inquiry and technological development in carbon utilization strategies, presenting a promising front in the battle against climate change.

Leave a Reply