As the global climate crisis intensifies, researchers at Case Western Reserve University have embarked on a vital mission to develop sustainable and efficient methods to convert waste into valuable products and fuels. Their groundbreaking research focuses on the conversion of carbon dioxide (CO2), a major greenhouse gas, into valuable chemicals using renewable energy sources. While the process of harnessing CO2 for industrial use has been a longstanding challenge, the team at Case Western Reserve University is on the brink of a breakthrough that could revolutionize waste conversion technologies.
The Quest for Sustainable Waste Conversion
The urgency to develop technologies for capturing and converting CO2 from waste or the atmosphere has never been more critical. Professor Burcu Gurkan, from the Case School of Engineering, stresses the need for benign conditions in CO2 conversion processes. The existing methods rely on high pressures, elevated temperatures, and specialized materials, making them energy-intensive and limited in efficiency. The need of the hour is an energy-efficient and sustainable process that can convert CO2 into valuable commodities.
Previous research efforts have predominantly focused on catalyst materials and understanding the energy-intensive CO2 conversion in water-based electrolytes. However, these water-based systems have limited CO2 capacity and often give rise to undesirable side reactions, such as hydrogen gas emissions. In a recent study published in the European journal Angewandte Chemie, researchers at Case Western Reserve University unveiled a breakthrough: the use of ionic liquids to effectively capture and convert CO2 in an electrochemical process.
Unlocking the Potential of Ionic Liquids
Ionic liquids, which are salts that melt below 100°C, have emerged as a game-changing solution in CO2 conversion. The ionic liquids developed by Gurkan’s team at Case Western Reserve University are unique in their ability to remain liquid at room temperature while also boasting high CO2 capture capacity and electrochemical stability. This crucial combination enables the desired electrochemical process, addressing the limitations of water-based systems.
The Role of Ionic Liquid Electrolytes
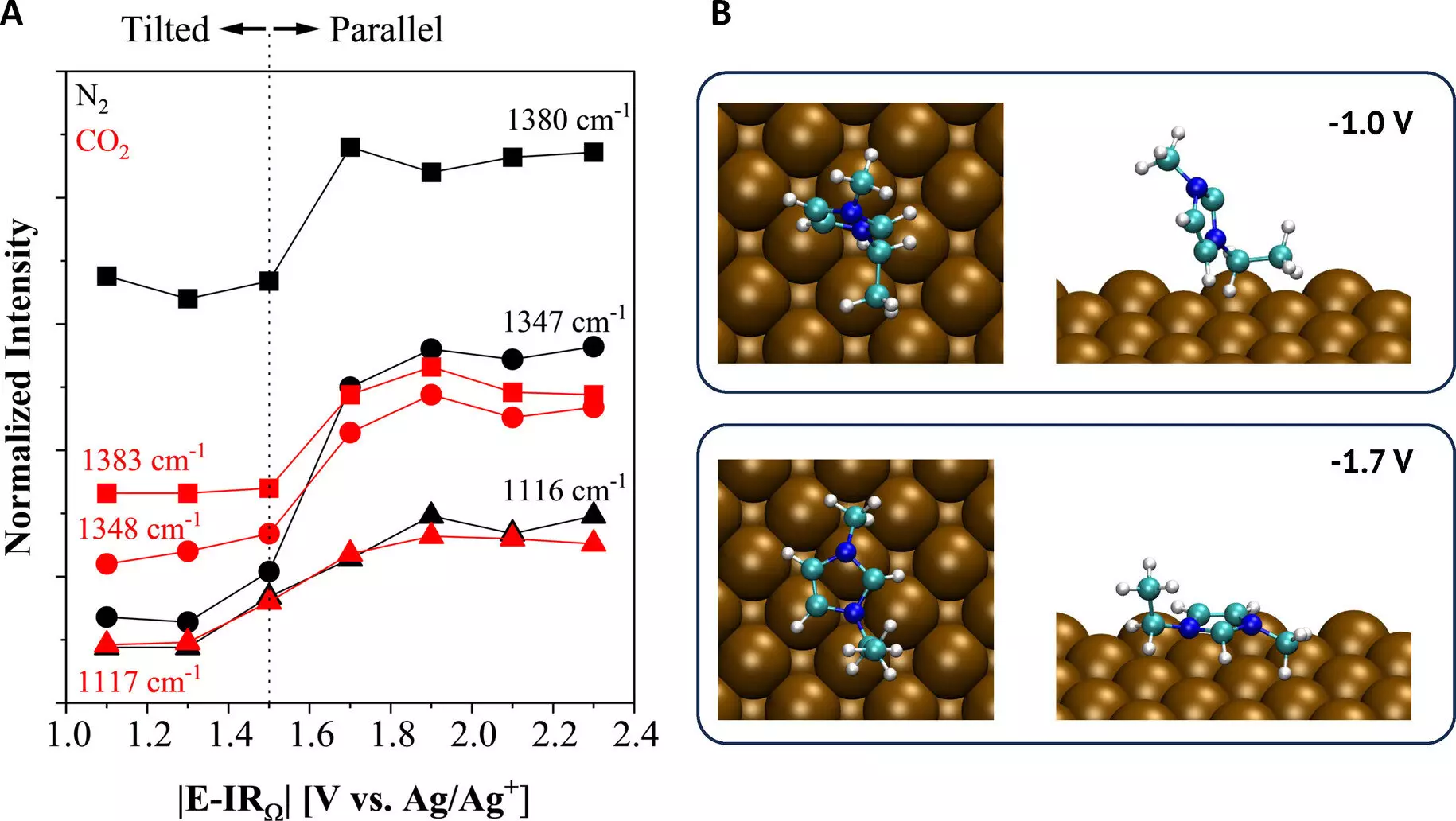
The team’s research, spearheaded by Oguz Kagan Coskun, a doctoral student in Gurkan’s group, delved into the fundamental mechanisms required for ionic liquids to activate the CO2 reduction reaction at the copper electrode surface. Through a combination of spectroscopic and electroanalytical techniques, they acquired valuable insights into the thermodynamics and product distribution of the reaction environment. This knowledge could pave the way for tailored designs of ionic liquids, allowing for better control and optimization of the CO2 conversion process.
One of the most significant outcomes of the research is the reduced energy requirement for driving the CO2 conversion reaction. By employing ionic liquid electrolytes, the team managed to decrease the energy input while simultaneously increasing the efficiency of the process. This development opens up possibilities for a wide range of industrially-relevant products without the presence of unwanted side products typically associated with traditional electrolysis methods.
The comprehensive nature of the study enables a deeper understanding of reaction environments, especially in unconventional electrolytes. By expanding their research to explore individual reaction steps further, the team aims to inform future electrolyte designs. Through this ongoing pursuit, they strive to enhance control over the chemical output of the process, thereby advancing the electrochemical approaches to CO2 recycling.
The groundbreaking research conducted by Case Western Reserve University is poised to transform the field of waste conversion by offering a sustainable solution for utilizing CO2. The development and utilization of ionic liquids as electrolytes in the electrochemical process have shown immense potential for harnessing CO2 for valuable chemical production. With further advancements and optimization, this technology could pave the way for a greener and more sustainable future, where waste is transformed into valuable resources. The work being done by the dedicated researchers at Case Western Reserve University holds the promise of a significant paradigm shift in waste conversion and CO2 utilization.

Leave a Reply