The challenge of tackling climate change has catalyzed intense research into methods to reduce carbon dioxide (CO2) emissions, which primarily arise from energy production, transportation, and various industrial processes. Among the innovative solutions being explored, electrochemical reduction has emerged as a viable strategy to transform captured CO2 into useful fuels and chemicals, such as methanol and ethanol. However, developing efficient catalysts for this process has been a significant obstacle. Fortunately, recent advancements led by a team from the U.S. Department of Energy’s Brookhaven National Laboratory offer a glimpse of hope and show promise for improving catalytic speed dramatically.
Electrochemical reduction utilizes electrical energy to convert CO2, a greenhouse gas, into other chemical compounds, effectively recycling it into valuable materials. This process involves the use of catalysts—substances that expedite chemical reactions without being consumed in the process. A primary hurdle in implementing electrochemical reduction at scale is finding catalysts that require minimal energy while performing efficiently enough to be economically viable. A recent study, published in the Journal of the American Chemical Society, presents a breakthrough in this area.
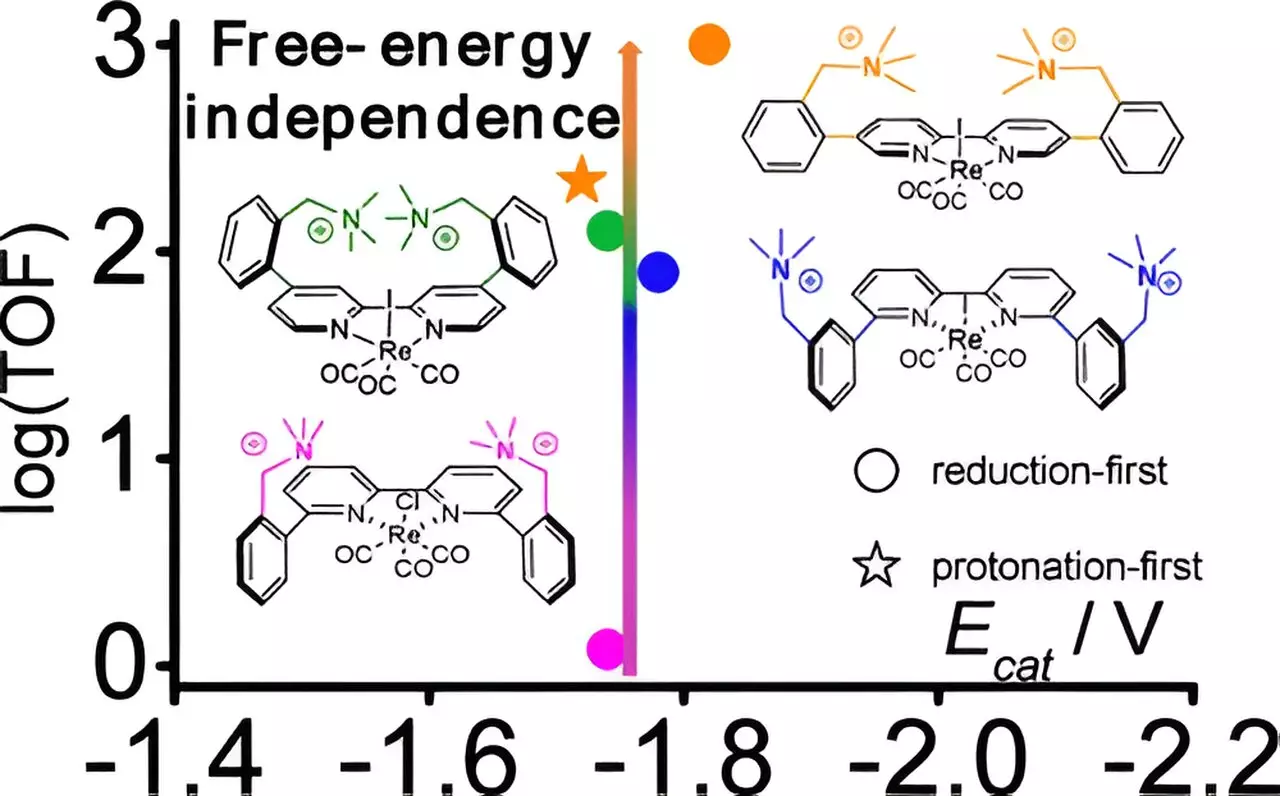
The collaborative research team, which included experts from Brookhaven, Yale University, and the University of North Carolina at Chapel Hill, unlocked significant improvements in the performance of rhenium-based catalysts. Lead researcher Gerald Manbeck explained that while many materials can catalyze CO2 reduction, they often demand high energy inputs, which poses barriers to large-scale applications. The innovative catalyst developed by this team requires considerably less energy, consequently promoting its future use.
The research team introduced three new variants of an existing rhenium catalyst by modifying it with positively charged cations. Through meticulous adjustments in spacing between these cations and the metal center, researchers observed an incredible surge in catalytic activity—an increase by a factor of 800. This enhancement did not necessitate substantial additional electrical energy, marking a crucial advancement in electrochemical processes.
Exploration of the mechanisms underlying the observed catalytic effects revealed that the distance between the cations and the rhenium center distinctively influences the reaction. Computational chemistry played a pivotal role, allowing researchers to delve into the stabilization effects exhibited by the cations during critical stages of the catalytic reaction. These findings indicate that the most efficient catalyst paved a novel low-energy pathway that deviates from traditional rhenium-based catalysis, which is often less efficient.
The avenues of exploration utilized various experimental methods, including cyclic voltammetry and infrared spectroelectrochemistry, to ascertain energy profiles and reaction kinetics. By employing a novel apparatus designed by some participating team members, the researchers could effectively observe minute chemical alterations occurring at the interface where catalytic reactions and energy supply converge.
The continued research trajectory set by the Brookhaven-led team promises even more innovative developments. Their next objective involves integrating semiconductor light absorbers, such as silicon, into the catalytic system. This integration aims to harness incoming light energy to partially drive the electrochemical reaction, thereby diminishing the reliance on direct electrical inputs. By aligning this approach with their mission to create efficient photoelectrodes, the researchers seek to harness solar energy, facilitating the conversion of CO2 and water into sustainable fuels.
The implications of this research extend beyond academic interests; they hold substantial potential to revolutionize how industry approaches CO2 emissions. As regulatory standards become more stringent and demand for sustainable practices rises, the findings of this study present a pathway toward lower carbon footprints while also creating energy-dense fuels. By enhancing the efficiency of CO2 reduction processes, industries can actively contribute to mitigating climate change, driving a shift toward a more sustainable energy future.
The fascinating developments in electrochemical catalysts, particularly those emanating from the collaborative efforts at Brookhaven National Laboratory and partner institutions, represent a significant leap forward in addressing global warming by transforming CO2 emissions into valuable commodities. Such pioneering research not only illustrates the power of scientific collaboration but also highlights the urgent need for continued innovation in the face of climate adversity. With sustained focus and resources, the practical application of these technologies may soon emerge on a global scale, offering a promising solution to one of humanity’s most pressing challenges.

Leave a Reply