In the quest for sustainable energy solutions, hydrogen has emerged as one of the most promising alternatives. Despite its potential as a clean fuel source, widespread adoption has been hampered by storage issues. Compared to traditional fuels like gasoline, hydrogen occupies significantly more space, causing a bottleneck in the development of practical applications. The need for efficient, lightweight storage solutions has led researchers to innovate various methods, culminating in a breakthrough from a collaboration of chemists at the University of Hong Kong, Northwestern University, and Duke University.
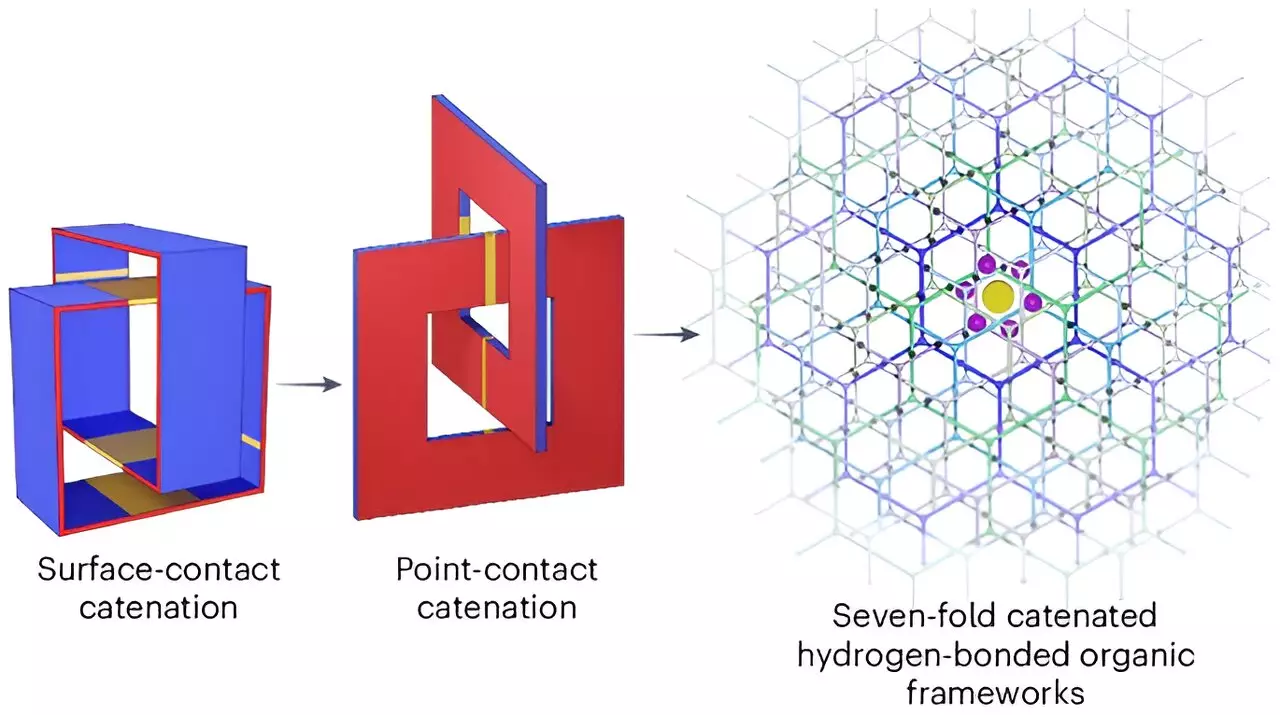
A recent study published in the revered journal Nature Chemistry showcases the innovative approach undertaken by this interdisciplinary research team. They have engineered a new supramolecular material specifically designed for compressing hydrogen with remarkable efficiency. The crafted material features a porous organic crystalline structure, strategically arranged to create interlinked honeycomb formations. This design facilitates optimum hydrogen storage by providing pores aptly sized for hydrogen molecules to bond securely.
This collaborative work aligns with the targets set by the U.S. Department of Energy, focusing on two critical performance criteria: the capacity to store at least 50 grams of hydrogen per liter, and ensuring the aggregate weight of the hydrogen remains below 6.5% of the storage medium’s total weight. Historically, achieving these goals has proved elusive for researchers; however, preliminary results from this new material are promising.
The testing phase of the new crystalline material yielded impressive outcomes, successfully storing 53.7 grams of hydrogen per liter and ensuring that hydrogen comprised 9.3% of the entire system’s weight. This performance not only meets but exceeds the established benchmarks. The integration of closely linked organic molecules enhances the material’s stability and reduces the issues associated with permeability that other materials have faced in similar experiments.
The researchers highlight that the strength of the hydrogen bonds with the crystalline structure is key to maintaining stability during storage. This level of efficiency presents a significant advancement over previous methods, suggesting that the developed material could soon play a role in innovative energy solutions.
Despite these advancements, the research does not come without its challenges. One of the primary concerns is the required cryogenic cooling for the system to function efficiently. This cooling method can be both bulky and costly, raising questions about practicality for commercial deployment. For large-scale energy solutions, these factors could hinder the viability of this breakthrough, necessitating further research to develop a more user-friendly approach.
The development of this supramolecular material by an esteemed team of researchers signifies a crucial step towards overcoming hydrogen storage limitations. Although challenges remain, particularly regarding cooling technologies, the findings represent a noteworthy leap forward in achieving sustainable hydrogen energy solutions. As further research continues, the potential for applying these innovative materials in real-world scenarios remains an exciting prospect for the future of clean energy.

Leave a Reply