The chemical industry stands at a crossroads, poised between the pressing demands for more sustainable practices and the need for continued innovation in the synthesis of essential compounds. Recent research from Kyushu University highlights a promising avenue for achieving this balance by utilizing microwave technology to enhance the conversion of biomass into olefins—a crucial chemical building block for various products, including plastics and pharmaceuticals. This innovation not only addresses the growing environmental concerns associated with traditional chemical synthesis methods but also enhances energy efficiency, which is increasingly vital in an era marked by climate change.
Historically, the production of olefins and other complex organic compounds has heavily relied on the reforming of naphtha, a process that is notoriously energy-intensive and contributes to significant carbon emissions. Researchers have been exploring alternatives, such as utilizing cooking oil waste and microalgal oils for catalytic cracking, which promises a more sustainable approach. However, the process remains hampered by high operational temperatures—typically between 500 and 600°C—that not only consume vast amounts of energy but also lead to the detrimental buildup of coke, which shortens the lifespan of catalytic materials.
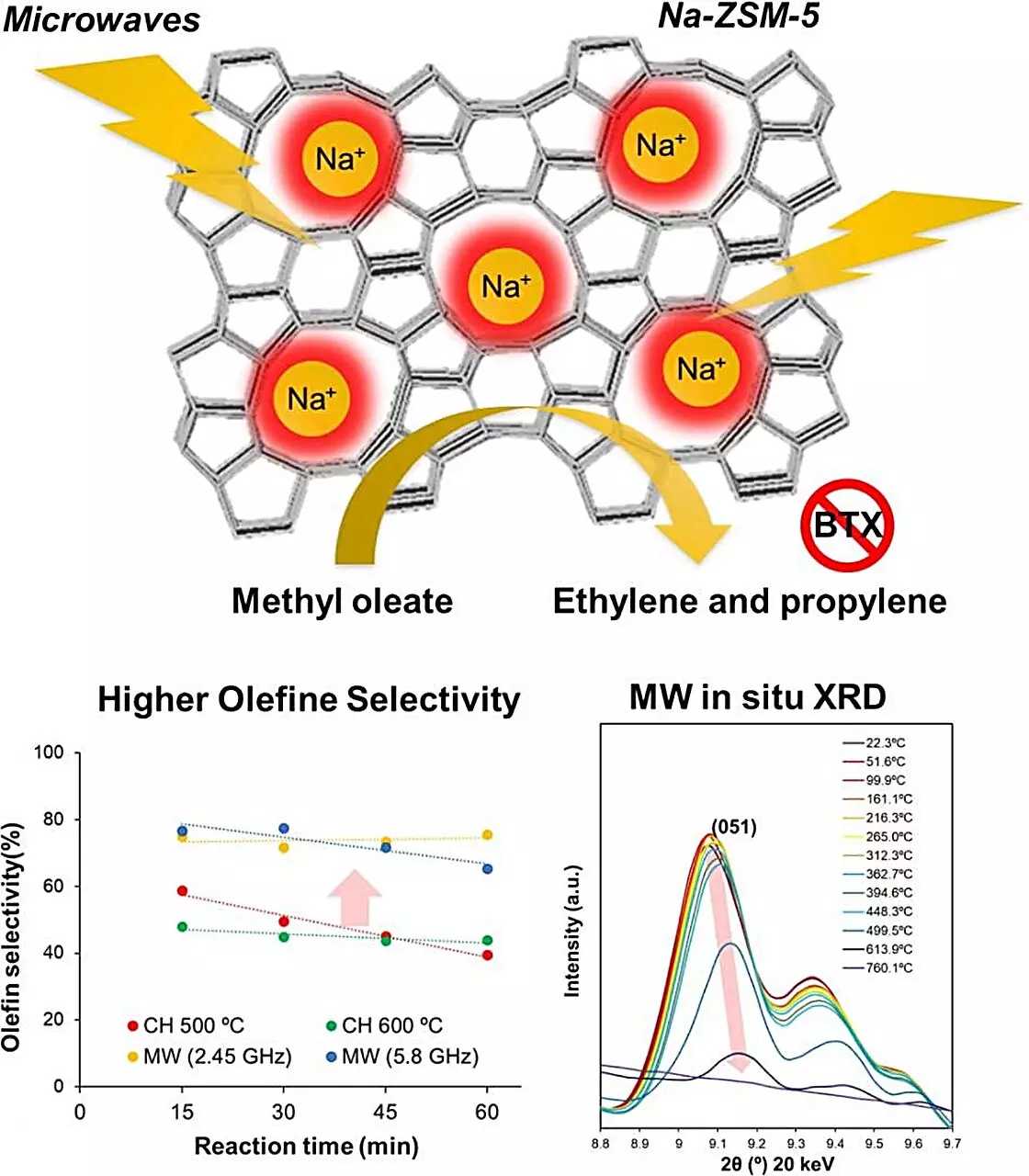
The breakthrough reported by Associate Professor Shuntaro Tsubaki and his team introduces microwave heating as a game-changing method to energize zeolite catalysts. Unlike conventional heating that relies on external heat transfer, microwaves interact directly with the materials, resulting in energy that can be precisely delivered to the catalyst. This innovative approach significantly reduces energy consumption while eliminating the drawbacks associated with conventional heating methods. The study revealed that microwaves are capable of generating localized “hot spots” within zeolite structures, enhancing gas-solid catalytic reactions without leading to coking.
In their rigorous examination, the researchers evaluated various zeolite substances to identify candidates that could respond favorably to microwave treatment. Na-ZSM-5, a sodium-based zeolite, was selected for its promising catalytic performance after extensive theoretical simulations and experimental validation. The comparative studies against conventional heating methods unequivocally demonstrated that Na-ZSM-5, when subjected to microwave energy, provided exceptional conversion efficiencies of fatty acid esters into olefins—an immense advantage in the context of sustainable chemical production.
Microwave-assisted catalytic conversion streamlined the synthesis process, achieving impressive results. Remarkably, Na-ZSM-5 facilitated olefin production with reductions in carbon dioxide emissions to just 1.3%, and there was no generation of carbon monoxide, a toxic byproduct of incomplete combustion. Most notably, the study found that microwave heating at 500°C yielded four times more olefins compared to traditional heating methods at the same temperature—evidence that the approach not only works more efficiently but is also far superior in terms of the quality of products generated.
The underlying mechanisms of this enhancement are particularly fascinating. Researchers examined the zeolite’s structural responses to microwave exposure and uncovered that localized heating resulted in temperatures in the zeolite lattice exceeding 1000°C, while the bulk material remained stable at 500°C. This phenomenon is believed to play a crucial role in promoting the desired olefin production by optimizing the reaction pathways and minimizing the occurrence of side reactions.
The implications of this research extend far beyond the laboratory. The findings illuminate a path towards integrating renewable energy sources—such as solar and wind power—into the chemical synthesis processes, thereby significantly reducing the environmental footprint of producing fundamental chemicals. This aligns seamlessly with broader sustainability goals that the global community is striving to achieve.
The team at Kyushu University is not resting on their laurels; efforts are underway to further improve microwave-driven catalytic processes. Their objective is to enhance both yield and energy efficiency while also scaling these processes for larger production capacities. Should they succeed, we could well witness a transformative shift in sustainable chemical production—heralding an era where the synthesis of essential chemicals can be conducted with minimal waste and a markedly lower carbon impact.
The innovative use of microwave technology in catalysis, as demonstrated by the research conducted at Kyushu University, marks a significant milestone for the chemical engineering industry. By harnessing this advanced heating method, researchers are not only contributing to the efficiency of biomass conversion into vital chemical precursors but also paving the way for a more sustainable and environmentally conscious future in chemical manufacturing. This research not only accentuates the potential of microwave heating but also inspires ongoing exploration into sustainable practices that align with the pressing challenges facing our planet today.

Leave a Reply