The industrial process for the conversion of methane to methanol has long been known to be extremely energy and resource-intensive. Scientists have been searching for more efficient and sustainable ways to carry out this conversion process, leading to the development of various catalyst systems over the past decade. However, many of these catalysts rely on rare and expensive transition or noble metals, making them less feasible for large-scale industrial applications.
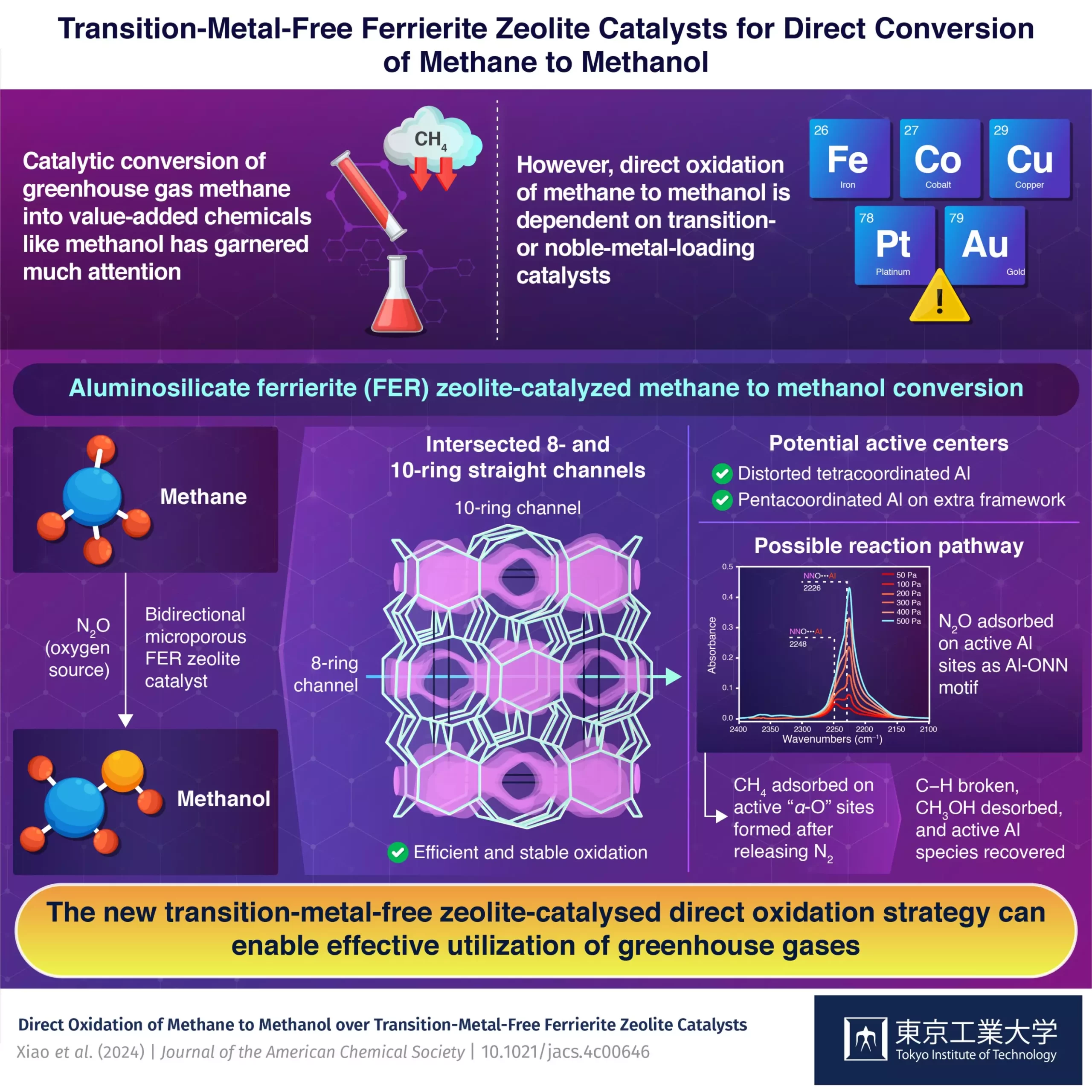
A recent study conducted by a team of researchers at the Nanospace Catalysis Unit of the Institute of Innovative Research at Tokyo Institute of Technology has unveiled a groundbreaking solution to the existing problem. Led by Associate Professor Toshiyuki Yokoi and Assistant Professor Peipei Xiao, the researchers have found that transition-metal-free aluminosilicate ferrierite (FER) zeolite could be used as an effective catalyst for the direct oxidation of methane to methanol.
The unique structural properties of FER zeolite, including its 2-dimensional structure with 8-ring and intersected 10-ring channels, make it highly stable towards chemical and thermal treatments. By taking advantage of these properties, Yokoi and his team were able to demonstrate the direct oxidation of methane to methanol using nitrous oxide as the source of oxygen. This breakthrough was published in the Journal of the American Chemical Society on April 10, 2024.
The researchers conducted various tests, including Fourier transform infrared spectroscopy (FTIR) and magnetic resonance spectroscopy, to understand the active sites in the zeolite catalyst. They found distorted tetracoordinated Al in the framework and pentacoordinated Al in the extra framework, which were identified as potential active centers for the conversion process. Through further experiments, the team mapped out the reaction pathway and achieved a methanol production rate of 305 μmol g-1 min-1 with 89% methanol and 10% dimethyl ether selectivity.
The development of transition-metal-free zeolite catalysts for the direct oxidation of methane to methanol represents a significant advancement in the field. It not only offers a more sustainable and cost-effective alternative to traditional catalyst systems but also provides a pathway for reducing greenhouse gas emissions and generating value-added materials from unwanted raw materials. The research conducted by Yokoi and his team paves the way for the utilization of methane and nitrous oxide in the production of valuable chemicals, opening up new possibilities for environmental and economic benefits.

Leave a Reply