The interactions between phosphorous acid and the platinum catalyst in high-temperature PEM fuel cells have been found to be more intricate than previously believed. Recent experiments at BESSY II using tender X-rays have revealed multiple oxidation processes at the platinum-electrolyte interface. These findings highlight the influence of variations in humidity on these processes, which can ultimately lead to improved lifetime and efficiency of fuel cells.
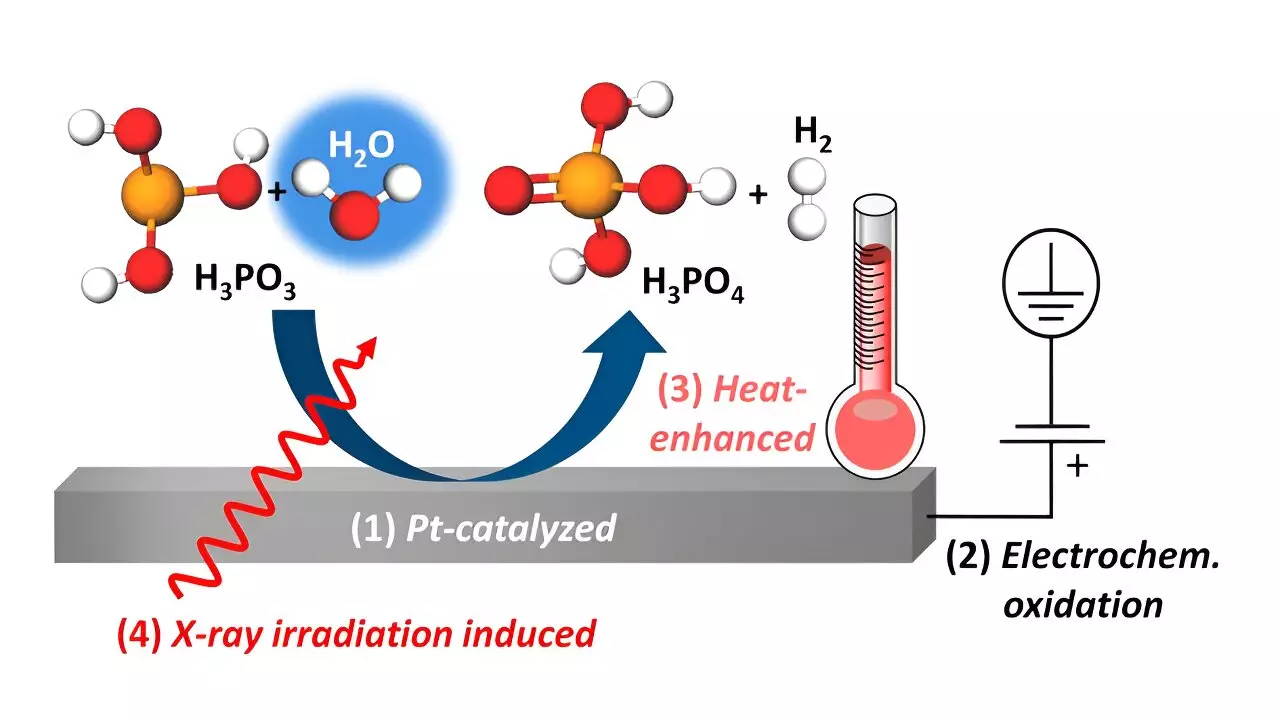
High-temperature polymer electrolyte membrane fuel cells (HT-PEMFCs) are becoming increasingly popular as micro-stationary electricity sources due to their ability to convert chemical energy from hydrogen into electrical energy. However, one of the drawbacks of these fuel cells is the leaching of phosphoric acid (H3PO4) proton conductor from the H3PO4-doped polybenzimidazole membrane, which can poison the platinum catalyst. Moreover, studies have shown that H3PO4 reduction to H3PO3 during operation can further deteriorate the performance of the platinum catalysts.
Researchers, led by Prof. Dr. Marcus Bär, have delved deeper into the interactions between platinum catalysts and H3PO3/H3PO4 by conducting experiments with a heatable electrochemical cell compatible for in situ tender X-ray studies. By utilizing X-ray absorption near-edge structure spectroscopy (XANES) at the P K-edge, the team monitored oxidation processes from H3PO3 to H3PO4. The results revealed various pathways for this oxidation reaction, including platinum-catalyzed chemical oxidation, electrochemical oxidation under positive potential bias at the platinum electrode, and heat-promoted oxidation.
One of the key findings of the study is the significant influence of humidity inside the fuel cell on the oxidation processes. The researchers suggest that controlling the humidification could help oxidize H3PO3 back to H3PO4, thereby preventing catalyst poisoning and enhancing the efficiency of HT-PEM fuel cells. These adjustments to the operating conditions of fuel cells could prove to be crucial in mitigating degradation pathways and advancing towards an H2-based energy supply. Prof. Dr.-Ing. Marcus Bär emphasizes the importance of this work as a step towards a more sustainable energy future.
The complex interactions between phosphorous acid and the platinum catalyst in high-temperature PEM fuel cells have been elucidated through in-depth research and experimentation. By uncovering multiple oxidation processes and understanding the role of humidity in these reactions, researchers have identified potential avenues for improving the efficiency and longevity of fuel cells. This study not only sheds light on the challenges faced by HT-PEMFCs but also provides valuable insights for optimizing the operational conditions of these energy sources. As we continue to strive towards a cleaner and greener energy landscape, studies like these play a crucial role in paving the way for a sustainable future.

Leave a Reply